Read The Root of Thought Online
Authors: Andrew Koob
The Root of Thought (8 page)
FIGURE 5.1 Calcium in action

In the field of biology, the removal of tissue cells from animals and subsequently culturing them in a Petri dish is a common practice. Cells adhere to each other like in tissue when they are placed in a culture dish.
Just as human beings are social creatures who need other human beings, so cells need other cells, a process that relies entirely on calcium. Without calcium, the cells are unable to grow as quickly and their structures change. They isolate themselves and eliminate cell interaction.
Extracellular calcium is 20,000 times higher than intracellular calcium. However, the cell has internal complexes that also store calcium at 10–50 times the rate of the surrounding intracellular space. The wide disparity of calcium concentrations inside and outside the cell is due to the interactivity of calcium when it is not controlled. Most calcium inside the cell is attached to proteins and sequestered in internal cell complexes. On the planet earth, calcium cannot exist freely without interacting with something. Like the most respected member of a family, all important matters in the cell involve calcium.
Further calcium experiments were performed on muscle after Ringer’s famous experiments. Extracellular calcium can stop muscle contraction. When it drops below a certain level, muscles twitch uncontrollably. Calcium is also stored in neurons at the synapse. When the electrical signal reaches the end of the axon, calcium floods the synapse from the extracellular space like people into a store for a 50 percent off sale. The calcium “flux” causes a release of calcium from sequestered stores inside the cell and is required for transmitter release. Because the understanding of calcium in the cell came about later than Hodgkin and Huxley’s squid giant axon experiments, the belief is that calcium contributes to the electrical gradient more than previously expected.
In astrocytes, the main signaling comes from complexes inside the cell called “internal calcium stores.” Neurons require the electrical axon impulse for calcium influx from the extracellular space to enter at the synapse and affect transmitter release. External calcium influencing transmitter release has been shown to come from astrocytes.
In the short paper published by Murphy and colleagues, which showed that transmitters can institute a calcium increase in astrocytes, they were concerned with a single cell and did not understand the extent of astrocyte reception of neuronal communication. A neuron signaling to an astrocyte could carry information from our senses. If neurons are the only cell involved in mental processing, why would astrocytes respond to the sensory stimulus of neurons? At the point of their discovery, Murphy and his colleagues did not understand that astrocytes might also be able to signal to other astrocytes and to neurons themselves.
A couple papers from Yale, the first by Ann H. Cornell-Bell and colleagues in 1990, demonstrated that calcium waves occur in astrocytes when they are stimulated. After a transmitter acts on an astrocyte or a researcher mechanically stimulates the cell with an electrode—a long plastic tip about 1/200th of a millimeter wide—the calcium wave spreads from one astrocyte to the next. The spread is similar to what Golgi described—through physical connections between the star-like cells long arms—similar to billions of octopi holding hands.
The understanding of how the cells held hands began in 1969. Researchers at the National Institutes of Health, Milton Brightman and Thomas S. Reese, laid the foundation for what would be known as the gap junction. Looking at pictures taken with the electron microscope, they demonstrated that astrocytes—with the ends of their processes probing blood vessels and neuronal surfaces—also come into contact with other astrocytes. It is now known that the end feet of different astrocytes wrap around each other and form a junction through which receptors bind the two cells.
Gap junctions are present in other types of organs as well, such as the liver and the heart. Gap junctions are fused together by intracellular channels. Imagine a lock and a dam. The water level on one side is higher than another, but a free exchange of barges and boats occurs in the lock where water levels can be raised or lowered to the other side’s level. The regulated lock and dam of the astrocyte gap junction determines what can pass from cell to cell.
About 230 gap junctions connect a pair of astrocytes in the cortex. If a dye is injected into one astrocyte, 50–100 neighboring astrocytes will be stained with the dye. In different brain regions, the degree of astrocyte cell-to-cell connection differs. Almost all cortical astrocytes are physically connected, as Golgi suspected for a cell responsible for thought.
Oligodendrocytes, the cells that wrap insulation around axons to better help neurons conduct electricity, also form gap junctions with astrocytes. Although they are not as efficient as astrocytes when conducting to each other, astrocyte gap junctions with oligodendroctyes might be a way to control myelination to increase conductance in neurons. This analogy can be better explained if one considers the oligodendrocytes as construction workers that work on the neural road. Astrocytes send an order
to fix the road because cars aren’t moving as fast as they’d like down the freeway.
In fact, in development of neurons, gap junctions can exist between astrocytes and neurons. These gap junctions can be understood as the building of a new road between astrocyte centers in the cortex—the architect and city planner building new infrastructure.
The main internal complexes where astrocytes store calcium are called the endoplasmic reticulum, mitochondria, and Golgi complex (named after the same Golgi, who noticed the structure in his stained cells in the late nineteenth century). These compartments are surrounded by membranes and have their own electrical gradient between the rest of the intracellular space of the cell. Because these compartments also exist in the intracellular space, the intracellular space outside these compartments is confusingly referred to as the cytosol.
The other paper from Yale, by Steven Finkbeiner in 1992, explained a series of experiments where they loaded dye into the astrocytes that attach to calcium. The visualized movement of calcium waves from one cell to the next occurs in a curvilinear pattern. One cell stimulates the next like a series of dominoes in patterns similar to the Milky Way.
Finkbeiner’s research blocked inositol triphosphate and was able to stop the flow of the calcium wave from one cell to the next.
It should be said here that inositol triphosphate is a painful and boring name concocted by chemists accoding to their naming system. It is not a protein or gene, but a molecule. For what it’s worth, inositol means it has 6 carbon atoms, 12 hydrogen atoms, and 6 oxygen atoms. The triphosphate means that three phosphate groups are attached. Some of the worst names in the history of science have come about as we enter the era of biology concerned with genes and proteins. We are subjected to the uniform naming of these poor structures, an idea that usually backfires as researchers realize the protein doesn’t do what they originally thought it did. A special place is in everyone’s heart for the guys that discovered a protein in the early 1990s and named it Sonic Hedge Hog after the Sega video game they were playing at the time. Even better is that it turned out to be incredibly important in embryonic development. There’s nothing more enjoyable than taking a class in biology taught by a difficult professor who is forced to say Sonic Hedge Hog 30 times in 15 minutes.
As it became known that calcium could signal from astrocyte to astrocyte, the implication of known calcium signal attributes became clearer. Calcium can also act on the genes in the astrocyte, affecting long-term changes in the cell’s reaction to calcium stimulus. Also, the endoplasmic reticulum, with calcium concentrations from 10–50 times higher than the other intracellular space (cytosol) is separated by its own membrane. Proteins are not sensitive to calcium binding in the endoplasmic reticulum as they are in the rest of the intracellular space because of the high concentration of calcium they are saturated with. In fact, binding of proteins to calcium is 1,000 times higher in the cytosol. This allows astrocytes to contain a ball of reactive calcium in their cells to be unleashed when stimulated. However, it’s hard to contain calcium.
For a forest that hasn’t seen a fire in a long time, striking a match will send it up in flames. However, the flooded endoplasmic reticulum is like the fire itself; proteins are unable to light if they are lighted already. The extremely high concentration of calcium in the endoplasmic reticulum and the sensitivity of proteins in lower levels of calcium cause constant pressure for the calcium to enter the cytosol. When it does, all hell breaks loose and binding occurs left and right. This short-term protein functioning can also develop into a long-term process, as calcium binding to proteins can change their long-term functions. The action of the actual wave in astrocytes is even more striking. The two types of receptors allow for calcium to leave internal storage sites. One receptor continually recruits more storage sites; the other is the inositol triphosphate receptor. It can be activated by calcium itself. So, as calcium moves in waves from cell to cell, it can continually recruit more calcium (see
Figure 5.2
).
FIGURE 5.2 A calcium wave starting from a single astrocyte in the middle of the frame (upper left) spreads outward to other astrocytes in the vicinity over the course of the next few seconds.
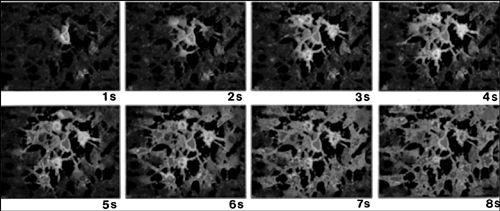
Reprinted from
Neuron
Vol. 6, Charles, A.C., Merrill, J.E., Dirksen, E.R., and Sanderson, M.J. “Intercellular signaling in glial cells: calcium waves and oscillations in response to mechanical stimulation and glutamate.” p. 985. Copyright (1991) with permission from Elsevier.
Blocking inositol triphosphate stops the wave. Inositol triphosphate receptor activation by calcium can desensitize the receptors. Inositol triphosphate is more sensitive to their receptors, but it is also less reactive than calcium and can act over many areas of the cell. Calcium and inositol triphosphate act like a hand on a faucet when they bind their receptors on the endoplasmic reticulum. After turning on the faucet, calcium flows out. The release of calcium from internal endoplasmic reticulum through these receptors results in sparks or puffs before it is sequestered back into the endoplasmic reticulum. These sparks or puffs can be amplified by neighboring endoplasmic reticulum as calcium itself releases more calcium stores.
To get calcium back into the endoplasmic reticulum, energy must be used. ATP (Adenosine-5-triphosphate) is produced by the mitochondria, the power plants of the cell, by using energy from the blood. ATP consumption in the cell is similar to world oil consumption. It is the voracious creation of energy and power. Believe it or not, the ion that stimulates ATP production in the mitochondria is none other than calcium.
When we eat and drink, our body breaks down our foods into energy. This energy is required for the mitochondria to produce ATP. Fats and sugars are broken down into units that are eventually utilized by the mitochondria transport system. When there is not enough energy available, calcium is unable to pump back into the endoplasmic reticulum and calcium diffusion can cause cell death. However, too much energy is stored in our body as fat.
During development, calcium channels on the outside of glial cells are expressed when the cells are growing and differentiating. When the cells reach maturity, the outside channels are removed, like training wheels on a bike. The astrocyte can now control calcium itself and “ride the bike with no hands.” The main way of astrocyte signaling in mature cells is from calcium release of internal endoplasmic reticulum stores. Activation of extracellular astrocyte receptors by transmitters released from neurons creates the formation of inositol triphosphate in the cytosol that causes rapid endoplasmic reticulum calcium release.
When transmitters act on astrocytes, they create a series of calcium “puffs”—sparks that cause a calcium wave that diffuses the astrocyte, recruiting more endoplasmic reticulum and mitochondria through the cell, spreading through gap junctions to other astrocytes in a wave that can spread half a millimeter to all cells in its vicinity. Then more endoplasmic reticulum recruitment, calcium, and inositol triphosphate passage through gap junctions continue to create this incredible event from astrocyte to astrocyte.
