125 Physics Projects for the Evil Genius (26 page)
Read 125 Physics Projects for the Evil Genius Online
Authors: Jerry Silver

Figure 38-1
The buoyant force equals the weight of the boat plus its cargo. This can be predicted by determining the weight of the displaced water
.
The buoyant force on an object is given by the weight of the fluid it displaces.
The buoyant force exerted on a floating object equals the volume of that object that is submerged (m
3
) times the density of water (1000 kg/m
3
) times the gravitational acceleration (9.8 m/s
2
).
- Take a weight of known volume. Either calculate the volume using geometry or determine how much water it displaces. Measure the weight on a spring scale in the air. Immerse the weight in the water. How is the weight affected by being immersed in water? How does this compare with the weight of the water that would fill the volume of the submerged object?
- Predict how much weight a boat can support based on the weight of the water that it can hold. Test your prediction.
- Predict where a float line will be based on the volume contained below the float line set equal to the weight to be added to the boat.
A buoyant force is exerted on an object that is floating or submerged in a liquid. An object will float if it is less dense than the liquid it is floating in. The buoyant force exerted on an object floating in water equals the
weight of water
that would occupy the
volume
of the object that is
underwater
.
Cartesian diver
.
An object is barely floating in a bottle. In this project, you control whether it floats or sinks, just by applying pressure on the side of the bottle.
- 2-liter plastic soda bottle with a reclosable cap
- water
- 1 medicine dropper
- a small object such as a paper clip to fine-tune the weight of the medicine dropper
- Partially fill the medicine dropper with just enough water to establish
neutral buoyancy
, which is a condition where the medicine dropper does not sink, but also does not float in water. Test and adjust until the right amount of water is in the medicine dropper. Use the paper clip to increase the weight of the medicine dropper. - Put the medicine dropper in the soda bottle.
- Fill the bottle to the top with water.
- Cap the bottle securely.
- Observe what happens as you apply pressure to the sides of the bottle. What happens as you relieve the pressure?
When the side of the bottle is squeezed, the diver descends.
When the pressure is released, the diver returns to the surface.
Pressure on the side of the bottle is transmitted to the medicine dropper. The pressure reduces the volume of the medicine dropper. The buoyant force depends on the size of the medicine dropper. A smaller-volume medicine dropper has a smaller buoyant force and sinks.
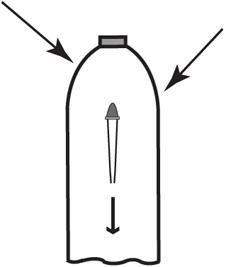
Figure 39-1
Cartesian diver
.
With the pressure reduced, the volume of the medicine dropper increases, leading to greater buoyant force. Greater buoyant force causes the medicine dropper to rise.
Neutral buoyancy occurs when the force of gravity equals the buoyant force resulting in equilibrium in the vertical direction. This is shown for a SCUBA diver in
Figure 39-2
. A diver would add weight to a belt as you did above with the paper clip to fine-tune the balance of forces.
Once you get the hang of this, you can put a “treasure” in the form of a small weight with a hook at the bottom of your bottle. You can then have your diver go down and try to recover the treasure.
Buoyancy depends on the volume of a submersed object. Pressure exerted externally reduces the volume of a submersed object, which, in turn, reduces the buoyant force.

Figure 39-2
Vertical forces in equilibrium. Photo by Dr. Michael Dershowitz
.
An air-pressure fountain
.
This is a nice attention-getting demonstration that produces a fountain-like spray in an inverted flask. If you are doing this as a demonstration—especially for younger children—be prepared to be asked to do it again.
- ring stand with a small 2-inch diameter ring
- flask (250 ml works)
- 1-hole rubber stopper to fit the flask
- approximately 12-inch section of glass tubing that can be inserted into the stopper
- hotplate
- oven mitt
- beaker of equal or larger volume as the flask
- water (with food coloring optional)
- safety glasses
1. Put on safety glasses.
2. Carefully slide the glass tube through the stopper, so approximately 1 inch protrudes through the narrow end of the stopper. Use proper techniques for handling the glass tubing (including wearing eye protection, protecting your hands as you push it through using a towel, and lubricating the edge with a bit of Vaseline, so you don’t have to force it through the hole).
3. Insert the stopper into the flask.
4. Attach the ring on the ring stand high enough so the entire length of the tubing is supported about ½ inch above the base of the stand.
5. Fill the beaker close to the top with water. (Food coloring can temporarily stain your fingers.)
6. Assemble the apparatus, as shown in
Figure 40-1
. Make sure everything fits and is secure.

Figure 40-1
Air pressure fountain
.
7. Take the flask out of the ring stand and remove the stopper with the tubing.
8. Put (a few tablespoons of) water into the flask. Set the stopper on a table.
9. Place the flask on the hotplate (
Figure 40-2
).
10. When the water starts to boil and the flask fills with steam (using the oven mitt), remove the flask from the hotplate and attach the stopper.
11. Quickly, but carefully (still using the oven mitt), reassemble the apparatus. One convenient way to do this is to note the position of the ring, remove the ring, and then place the ring over the collar of the flask. Then, with the stopper inserted, invert the flask, and (with the flask supported by the ring as it is transferred) reattach the ring on the stand. It wouldn’t hurt to choreograph this a little bit before doing it (and have a second person help you). The idea is to do the transfer quickly (so you don’t lose all of your steam), but
safely
(because you are working with glass and hot liquids). Placing an ice cube on top of the flask may further accelerate the process.

Figure 40-2
Heat a small amount of water in the flask
.
Expected Results12. With the flask in the ring stand and the glass tubing close to the bottom of the beaker, observe what happens.
At first, the water starts to rise up the tube. This begins slowly at first. As the water works its way up the tube, it begins to pour into the flask. Once the water touches the interior of the flask, it begins to spray, forming a fountain that increases in intensity until the water is completely drawn out of the flask (
Figure 40-3
).
If positioned just right, the fountain ends in a gurgling effect. While many observers may expect the rise of the liquid up the tube, the surge of the fountain catches many people off guard.

Figure 40-3
Air pressure causes the liquid to spray into the flask
.
As the steam inside the flask begins to cool, the air pressure inside the flask drops. This is primarily the result of the phase change of the steam from vapor to liquid water, which occupies a much smaller volume. The cooling air inside the flask also contracts, adding to the reduced pressure. Atmospheric pressure pushes down on the liquid in the flask, driving up into the glass tube. The cooler the flask gets, the lower the pressure. This process feeds on itself in an accelerating manner, producing the fountain effect.
The mechanism that drives the liquid up into the flask is the basis for what is known as a Torricelli barometer. Air pressure is measured by how high a column of water can be supported by air pressure with a vacuum in the flask. Mercury is used instead of water because standard air pressure can support a mercury column roughly 30 inches high, compared with a much-higher column for water. Because of potential difficulties in working with mercury in academic settings, it is probably best just to read about this one.