125 Physics Projects for the Evil Genius (25 page)
Read 125 Physics Projects for the Evil Genius Online
Authors: Jerry Silver

Which weighs more: an empty cup or a full cup? Obviously, the full cup. So, if you turn a cup upside down with a flat cover, which has the better chance of being supported: the (lighter) empty cup or the (heavier) full cup? Many people find the outcome surprising.
- cup or beaker with a flat, circular, smooth top. (If the beaker has a spout, it should be flat enough so it can make contact with a card at all points along its top surface.)
- water to fill the cup
- flat, stiff, lightweight square, large enough to cover the entire top surface of the cup. The square should not become waterlogged. Cardboard is not the best choice. Foamboard stands up to water better. Index cards can work, but you must be careful not to let them flex and break the seal with the cup.
- Start with the empty cup. Cover the empty cup with the square. Turn it upside down and observe what happens (
Figure 36-1
). - With the cup still empty, moisten the top surface of the cup to make it more sticky. Cover the cup and turn it upside down. What happens?
- Now fill the cup with water to the brim. It won’t hurt to have it go above the surface of the cup or to allow some water to spill out. Cover the cup with the square. Invert and observe what happens.
The square will fall off with the empty cup, even with the benefit of the surface being sticky. The water in the full cup, however, will be held in place by the square (
Figure 36-2
).
The water in a beaker 5 centimeters in diameter and 12.7 inches tall has a mass of 250 g (0.25 kg) and weighs 0.55 pounds (or 2.5 newtons). The air pressure on the 5 centimeter (roughly 2 inch) diameter circle is over 3 pounds. The air pressure is far greater than the weight of the water in the beaker. The empty beaker has the same pressure inside and outside the cup, so the air pressure is balanced and the weight of the cup causes it to fall. The adhesion of the square to the cup is clearly not enough to make up the difference.

Figure 36-1
With the cup empty, there is nothing to hold the square onto the cup
.
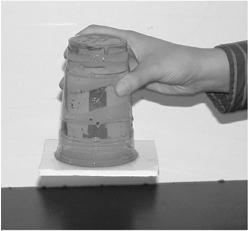
Figure 36-2
The force exterted by air pressure is greater than the weight of the water
.
Calculate how tall the glass can be with the card supported by air pressure. (The density of water is 1 g for every cubic centimeter and 1000 g is the equivalent of 2.2 pounds.) Atmospheric pressure will support a column of water 34 feet high. Since mercury is more dense than water, atmospheric pressure will support a column of mercury about 30
inches
high. The exact height varies with local air pressure and provides a way to measure changes in air pressure.
Air pressure is large compared to the pressure exerted by the weight of a cup of water.
Pressure. Sometimes the news can be pretty heavy
.
How much pressure does the atmosphere exert on a sheet of newspaper? As with the previous experiments, the force exerted by air pressure can be surprisingly powerful.
- section of newspaper
- table
- piece of wood about 12 to 24 inches long and roughly 1 to 2 inches wide. (The wood should be thin enough so it can be readily snapped in half by someone who is not a black belt. It should also be stiff enough so it will break rather than flex if stuck. A ruler that you are willing to dedicate to the cause of science usually works.)
- Place the piece of wood on the table, extending approximately one-half its length.
- Place a few layers of the newspaper over the wood. Lay it out so it is as flat as possible. Remove any “air pockets” that you can under the paper and make sure the edges are flat.
- Using your best Maxwell Smart karate chop, strike the wood. Hit it hard enough to break the wood. Show it no mercy (
Figure 37-1
).
Many people would expect the wood to push the paper up and throw the paper partway across the room. However, if the paper is properly sealed over the wood, striking the wood results in the wood being pinned to the table and breaking apart as if it were clamped to the table.
Let’s say you have a 1-inch wide ruler that extends 10 inches under the paper. This means that
147 pounds of air pressure
is pressing down on the ruler. About the same as a medium-sized person standing on the paper holding down the ruler. Air pressure is
that
strong. If this doesn’t work, it isn’t because of insufficient air pressure. The ruler may not break if: air leaks under the paper from the edges, the wood is too flexible to break, or the wood is too thick to break. It is unnecessary, of course, to actually break the ruler to demonstrate the strength of air pressure on the paper.
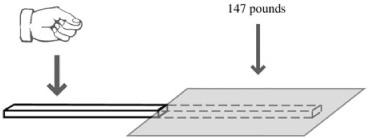
Figure 37-1
Breaking a board with only air pressure holding the other side down
.
Air pressure on paper can also be observed pressing down on the pages of a book. After interleaving the pages of two similar books, it will be very difficult, if not impossible, to pull the two books apart. This is not the result of the friction of the pages, but it is a direct effect of the air pressure holding the pages together.
The power of air pressure can also be demonstrated using a suction cup such as those used to pop out minor dents in car side panels. To do this you will need an object with a smooth surface. One good example is a laboratory stool.
1. Attach the suction cup to the top surface of the stool, as shown in
Figure 37-2
. Make sure the suction cup seals to the top surface of the stool.
2. Pull up on the suction cup.
The suction cup should be able to lift an average-sized stool up off the ground. There is a common misconception that a vacuum somehow pulls or “sucks” objects to it. This is not the case. Suction cups work because of a
difference
in air pressure between the outside of the suction cup and the little air trapped under the suction cup. The pressure on a suction cup that is 4 inches in diameter (assuming a perfect seal) would be greater than 150 pounds.
Air pressure exerts a force on a surface in proportion to its area.

Figure 37-2
Archimedes’s principle. What floats your boat
?
Does iron float? Clearly a cube of iron, which is much denser than water, will sink. Then, how is it possible for a boat made of iron (or iron alloy) to float? In this experiment, you investigate the forces that counteract the force of gravity to allow objects that are denser than water to float.
- “boat”: a shallow plastic cup (such as a ¼ pound coleslaw container). You can also use a piece of wood as your boat.
- “lake”: a plastic tray or fish tank filled with water deep enough and wide enough to float the “boat”
- “cargo”: small weights, pennies (each penny has a mass of 2.7 g)
- 100 mL graduated cylinder
- Measure the volume of the boat. You can do this by geometry, if you are so inclined, or you can do it by filling the cup with water and measuring the amount of water to do this.
- The number of grams of water that occupy the volume of the boat equals the number of grams of cargo it can carry just before it sinks.
- Test this by adding the amount of weight you predicted. Don’t forget to include the weight of the boat itself. If you use pennies, count each as 2.7 grams (or weigh them). As you add weight, be careful not to tip the boat or you will capsize it prematurely.
- Compare your prediction with the amount of cargo your boat could actually carry. See
Figure 38-1
. - We are taking a slight liberty here for the sake of clarity by focusing on the mass. What holds the boat up is a buoyant
force
, which is measured in newtons. The buoyant force equals the
weight
of the water (also measured in newtons) displaced by the floating object.
As a rule of thumb, for every 1 mL that an object is held submerged below the surface of the water, there is a buoyant force capable of supporting 1 gram of mass.
An equivalent way of expressing this is an object will float if the density of the entire boat, considering its entire volume, is less than the density of the same volume of water. The density of water is 1 gram/cubic centimeter or 1000 kilogram/cubic meter.
