A Step Farther Out (4 page)

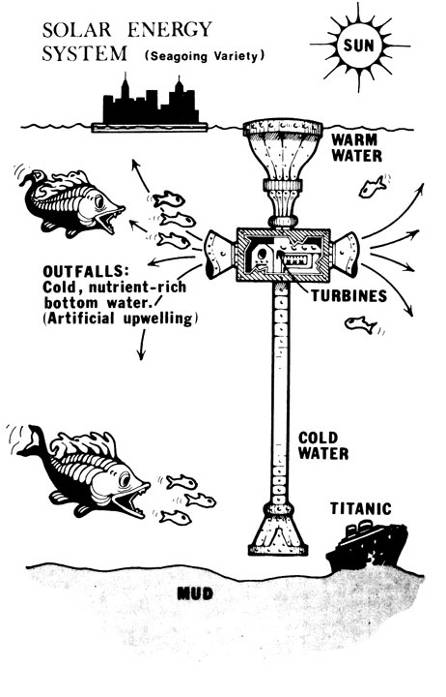
Certainly there are some engineering problems; but not so much as you might expect. The volumes of water pumped are comparable to those falling through the turbines at a large dam, or passing through the cooling system of a comparable coal-fired power plant. The energy itself can be sent ashore by pipeline after electrolysis of water into hydrogen and oxygen; or a high-voltage DC power line can be employed; or even used to manufacture liquid hydrogen for transport in ships as we now transport liquid natural gas.
As to the quantity of power available: if you imagine the continental United States being raised 90 feet, forming a sheer cliff from Maine to Washington to California to Florida and back to Maine; then pour Niagara Falls over every foot of that, all around the perimeter forever; you have a mental picture of the energy available in one Tropic, one band between the equator and the Tropic of, say, Cancer. It is more than enough power to run the world for thousands of years.
Finally the feasibility of OTS: in 1928 Georges Claude, inventor of the neon light, built a 20 kW OTS system for use in the Caribbean. It worked for two years. One suspects that what could be done with 1928 technology can be done in 1988.
OTS is not the only non-polluting system which could power the world forever. Solar Power Satellites would do the task nicely. SPS will be discussed in later chapters; but few doubt that they could provide more than enough energy to industrialize the world, and we understand how to build them far better at this moment than we understood rockets on the day President Kennedy committed us to going to the Moon in a decade.
___________
___________
That is a point worth repeating: we can power the Earth from space. We do not "know how to do it" in the sense that all problems are solved; but we do know what we must study in order to build large space systems. When John F. Kennedy announced that the United States would land a man on the Moon before 1970, the reaction of many aerospace engineers was dismay: not that anyone doubted we could get to the Moon, but those closest to the problem were acutely aware of just how many details were involved, and how little we had done toward building actual Moon ships. We had at that time yet to rendezvous or dock in space; there were no data on the long-term effects of space on humans; we had not successfully tested hydrogen-oxygen rockets; there were guidance problems; etc, etc. Thus the dismay: there was just so much to do, and ten years seemed inadequate time in which to do it.
Solar Power Satellites, on the other hand, have been studied in some detail; and we have the experience of Apollo and Skylab. We know that large structures can be built in space; they require only rendezvous and docking capabilities, and we've tested all that. We know we can beam the power down from space; the system has been tested at JPL's Goldstone, and the DC to DC efficiency was 85%. There are other problem areas, but in each case we know far more now than we knew of Mooncraft in 1961.
Ocean Thermal and Solar Power Satellites: either would power the world. I could show other systems, some not so exotic. My engineering friends tell me that OTS and SPS may even be the hard way, and there are much more conventional ways to supply Earth with energy.
No matter. My point is that
we can find the energy.
The method used is unimportant to the argument I make here: that we can survive, and survive with style.
Given energy we will not starve; we will lick the pollution problem; and we will generate the wealth which historically has brought about population limits. At least three of the dooms facing us can be avoided.
That brings us to the fourth doom: depletion of non-renewable resources. Can we manufacture the materials needed for survival with style? And can we do it without polluting the earth?
Surely we can. We can go to space to get the materials—and in doing it w£ can avoid pollution entirely. (There are, of course, those who worry about "polluting outer space", an example of non-quantitative thinking. Were we to devote the Gross World Product exclusively to the task and vaporize the Earth in the attempt we could not manage to pollute more than a fraction of a percent of the space in the solar system, and our effect would be temporary. One suspects that those who worry about "polluting outer space" are either incredibly arrogant, or actually are motivated by a desire for Zero Growth for its own sake.)
Metal production makes an excellent example. Mining and refining metals are some of the most polluting actions we manage, and metals are the most irreplaceable non-renewable resources we have. Give us enough iron and steel, copper, aluminum, zinc, and lead, and surely we'll have our problems licked. Give us enough metals and energy and we'll have wealth.
After all, it's mine tailings that produce some of the really horrible pollution; copper refineries that poison so many streams; and those belching steel mills that made Pittsburgh a legend (although Pittsburgh is also an excellent example of how pollution may be cleaned up once it is determined that cleanup has to be accomplished; a whole generation has never seen the smoke and fire of old Pittsburgh). Furthermore, processing metals uses up vast amounts of energy.
Give us metals free and clear, and the rest is easy. Give us enough metals and we'll industrialize the world. Besides, if we can do
that
in space, we can probably do anything else that has to be done. Consequently, I'll use metal production as my illustrative example.
___________
METALS FOR THE WORLD. . . .
In 1967, the United States produced 315 million tons of iron, steel, rolled iron, aluminum, copper, zinc, and lead.
Total metal produced, USA, 1967: 2.866 x 10
14
grams.
Assume 3% ore, of density 3.5 gm/cm
3
, and the USA produced the equivalent of a sphere 1.7 kilometers in diameter.
At 230,000,000 population, we produced 1.25 x 10
6
grams
per capita.
To supply the world with that much requires 5 x 10
15
grams or FIVE BILLION TONS.
Assuming 3% ore at 3.5 gm/cm
3
, five billion tons of ore is a sphere 2.25 kilometers in radius or 4% kilometers in diameter.
There are 40,000 or more asteroids larger than 5 km in diameter.
We may not run out of metals after all. . . .
___________
In 1967, a year for which I happen to have figures, the United States produced 315 million tons of iron, steel, rolled iron, aluminum, copper, zinc, and lead. (I added up all the numbers in the almanac to get that figure.) It comes to 2.866 x 10
14
grams of metal. Assume we must work with 3%-rich ore, and we have 9.6 x 10
15
grams of ore, or 10.5 billion tons.
It sure sounds like a lot. To get some feel for the magnitude, let's put it all together into one big pile. Assuming our ore is of normal density we end up with a block less than 1.5 kilometers on a side: something more than a cubic kilometer, something less than a cubic mile. Or, if you like a spherical rock, it's less than two kilometers in diameter.
There are 40,000 or more asteroids larger than 5 km in diameter.
We may not run out of metals after all. . . .
But—the title of this chapter is "Survival with Style." Style to me does not consist of the West as an island of poverty in the midst of a vast sea of misery. Style, to me, means that everyone on earth has a chance at wealth—at least at a decent life.
Can we not agree that if everyone on Earth had the
per capita
metal production of the US, we would probably have achieved world riches? Especially since we export much of ours to begin with; surely it's enough?
Thus we take our 315 million tons and multiply by the world population, then divide by the US population; assume 3% ore, and we find how much we'll need. The result works out to a sphere less than four miles in diameter—and there are well over 100,000 asteroids larger than that.
Three percent ore is no bad guess as to what they're made of, either. Actually, given the data from the Moon racks, 3% is an underestimate of the usable metal content of the average asteroid. We've had heavy nickel-iron meteorites fall that were nearly 80% useful metal. Then too, some of the asteroids were once differentiated—that is, they were large enough that metallic cores formed. Then over the last four billion years the planetoids got bashed around until a lot of the useless exterior rock was knocked away, leaving the metal-rich cores exposed where we can get at them.
___________
CALL SMYTHE, THE SMOOTHER MOVER. . .
Take one each, FIVE BILLION TON asteroid. Move from the Belt to Earth orbit.
Requires a velocity change of 7 kilometers a second.
KE = V
2
M V
2
or, we need 1.225 x 10
27
ergs.
For reference, the world annual energy use is 10
29
so we're using about 1% of it . . . .
That's also 30,000 megatons.
And 30,000 one megaton bombs might just do it.
For a slightly more efficient system, we can get the energy by converting 2,000 tons of hydrogen to helium . . .
Once we have the rock in Earth orbit, it's simple to get the metal out. We merely boil the entire rock. Of course that takes rather large mirrors, but what the heck. . . .
__________
Over 100,000 asteroids, each capable of supplying the world with more metal per person than the US consumes in a year. Surely we won't run out of metals—but can we use them?
Sure we can. First, for the moment let's forget that the asteroids are
way
out there in the Belt, and concentrate on how to get the metals out assuming we have the rocks in Earth orbit. That turns out to be easy. We can use sophisticated methods, but there's also brute force: boil the rock
It takes about 2000 calories per gram to boil iron. That's about the worst case for us, so we'll imagine the entire asteroid is made of iron. It takes, then, about 8.8 x 10 ergs, or twenty thousand megatons, to boil it all away.
The sun delivers at Earth orbit about 1.37 million ergs a second per square centimeter, and out in space we can catch that with mirrors. To boil our rock we could put up a mirror 80 kilometers in radius. That's too big; but we don't have to boil it all at once. A much smaller mirror to focus the sun onto a small part of the rock would be preferable.
A space mirror need be nothing more than the thinnest aluminized Mylar, spun up to keep its shape. There's no wind or gravity in space. A mirror one or two kilometers across is a relatively simple structure—and more than adequate for our job. If need be we can actually
distill
off the metals we want.
Note, by the way, that there's been absolutely no pollution of Earth so far—even though we've got metals for the entire world. All the waste is out in space where it can't hurt us. But we do have a problem. My metals are
not
in Earth orbit; they're out there in the asteroid Belt, and they've got to be moved here—and that's going to take
energy.
Let's see just how much it does take. To get from Ceres to Earth you need a velocity change of about 7 kilometers a second. By definition energy is mass given a velocity change, so we can quickly figure out how much; if we move the entire rock it comes to about 1% of the world's energy budget. That's not so much; we expend far more than that on metal production already.
To be more precise, it's about 60,000 megatons; and if need be, we can use hydrogen bombs. Put an H-bomb at the center of mass of an asteroid and light it off; I guarantee you that sucker will
move.
It's expensive, but not grossly so, assuming I have laser triggers for my H-bombs; only a few tons of hydrogen.
I could also do it with fusion: at 10% efficiency I get 6.4 x 10
17
ergs per gram of hydrogen, and I need about 10
27
ergs total to move the rock; for an engine I use an ion engine, breaking up parts of the asteroid for reaction mass. What arrives is something less than I started with, but who cares? What I'll throw away as reaction mass is the slag from my refinery.
(For those who haven't the foggiest notion of what I'm talking about: a rocket works by throwing something overboard. The reaction mass is what's thrown. Although the big space program rockets use gaseous exhaust as reaction mass, there's no reason you couldn't use dust, ground up rock, or slag from a metals refinery. It's all a question of whether you can throw it sternwards fast.)
But that leads to another possibility: why not set up the refinery out at the Belt? Put up vast mirror systems and do the refining on the way in; use the slag as reaction mass to move the whole works, rock, refinery, and all. I can power
that
with solar mirrors. Or I can do all at once: use bombs for initial impetus, set up mirrors when I'm closer in, and while I'm at it run a hydrogen fusion plant aboard the moving strip-mine/refinery/spaceship I have created.
At worst I have to carry about one Saturn rocket's worth of hydrogen, plus several shiploads of crew and other gear; and for that I get an entire
year's
worth of metals for the world. The value of my rock is somewhere near a trillion dollars once it's in Earth orbit; more than enough to pay for the space program and pay off the National Debt at the same time.