Beyond the God Particle (8 page)
Read Beyond the God Particle Online
Authors: Leon M. Lederman,Christopher T. Hill
Tags: #Science, #Cosmology, #History, #Physics, #Nuclear, #General

Further experiments indicated that the nucleus was indeed tiny—one-trillionth of the volume of the atom—even though most of the mass of the atom, more than 99.98 percent of it, resided in the nucleus. At the time of this discovery, within this tiny solar system model of an atom, all the classical laws of physics were still thought to be rock solid, just as in the macroscopic solar system with the sun and its planets. The same laws of classical physics were believed to work in the atom just as they did everywhere else—until Niels Bohr showed up.
THINKING THROUGH THE ATOM
Niels Bohr
10
was a young theoretical physicist from Denmark who was studying at the Cavendish Lab, and he happened to attend a lecture by Rutherford. He was immediately captivated by this new atomic theory of electrons orbiting nuclei. Bohr arranged to visit the great man for four months in 1912, while Rutherford, at the time, was working in Manchester. Sitting down and thinking about the new data, Bohr quickly perceived something profoundly significant about Rutherford's planetary model of the atom.
It was a complete disaster
, according to the known laws of physics!
Bohr realized that, in their state of rapid circular motion about the nucleus, electrons would radiate away all of their energy in the form of electromagnetic waves very quickly. Like the swoop of a seagull into the sea, the electron orbits would quickly shrink to zero, within a tenth of a millionth of a billionth of a second. The electrons would spiral down into the nucleus. This would make the atom, ergo all of matter, chemically dead and the physical world as we know it impossible. The exact classical equations of electricity, due to Maxwell and based upon Newtonian physics, spelled disaster for the atom. Either the model had to be wrong,
or the venerable laws of classical physics had to be wrong
.
Bohr applied himself to understanding the simplest atom—the hydrogen
atom—which would have a single electron in orbit around a positively charged nucleus consisting of a single positively charged particle called a proton. Thinking about the new quantum ideas that were in the air, that particles are also waves, young Bohr was led to propose a very novel idea. He argued that only certain special orbits can ever happen for electrons in atoms because the motion of electrons in these orbits must be like that of waves. These are like the natural wavelike motion of a ringing bell or a Chinese gong, with a dominant lowest tone, or
mode
, and a sequence of “overtones” or “harmonics.” The lowest mode, what we mostly hear when the bell tolls, would be the one with the least amount of energy, corresponding to the wave motion where the electron is moving closest to the nucleus. In this lowest orbit the electron cannot radiate away anymore energy, because this is the state of lowest possible energy for the electron motion—the electron has no lower energy state into which to go. This special orbit is called the
ground state
. This is one of the hallmarks of quantum theory: atoms cannot just collapse into nothingness and are actually supported by the quantum wave motion, leading to the existence of a ground state, the state of lowest possible energy.
In three papers published in 1913, Bohr articulated his audacious quantum theory of the hydrogen atom. Each of the atom's magic harmonic orbits is characterized by a certain energy. An electron emits a definite amount of radiation when it “jumps” from an orbit of higher energy down to one of lower energy. It emits a photon, the particle of light, whose energy is given by the difference of the energy of the two orbits. With billions of atoms doing this at the same time, we see bright and unique colors for the emitted light, the photons all having exactly the same energies. Bohr put his theory to work and calculated the wavelengths of all the emitted photons, the colorful “spectral lines,” seen in a spectroscope from hot glowing hydrogen gas. His formula worked perfectly! Electrons now moved in “Bohr orbits,” or “orbitals,” within the atoms.
None of this made the slightest sense in the familiar framework of Newtonian-Galilean physics. It required sweeping changes in our understanding of physics and the further development of the radical new ideas of quantum mechanics. In any case, atoms were indeed seen, by now, to be made of still smaller objects: the electrons and the atomic nucleus, and the rules of motion, the relevant laws of physics, were now totally new and totally quantum mechanical.
QUANTUM WAVES AND COSMIC RAYS
The quantum wavelike behavior of all matter was established within the first several decades of the twentieth century through numerous experiments, and the quantum theory was cobbled together (see
note 3
). By accelerating particles, we can shrink their quantum wavelengths. The overall characteristic “size of the atom” is determined by the “Bohr radius,” which is the size of the quantum wave of the electron ground state of hydrogen and is about 0.000000005 centimeters (0.5 × 10
–8
cm). The atomic nucleus is very much smaller, about one-hundred-thousandth the size of the Bohr radius. To explore these much smaller distances required much more energetic probes. High-energy particle accelerators would not arrive on the scene until the 1950s.
Nature, however, provides one extremely high-energy source of particles. These are very energetic cosmic rays that bombard Earth coming from deep outer space.
11
The cosmic rays are produced by violent processes in distant star systems, such as supernovae, pulsars, and active galactic nuclei. They are steered on the voyage to Earth by galactic magnetic fields, and their sources generally cannot be identified. The energies of cosmic rays extend way up beyond the highest energies of any particles we have ever seen or produced in the laboratory, higher than the LHC itself. The highest-energy cosmic rays ever detected have about 100,000,000,000,000,000,000 electron volts of energy (That's 10
20
eV; for comparison the LHC design energy is 14 trillion eV, or 1.4 × 10
13
eV), but these cosmic rays are extremely rare, only one of them passing through one square mile of sky every century! However, cosmic rays of energies up to about 1,000,000,000,000 eV (1,000 GeV; that's 1,000 Giga-eV, and 1 Giga = 1 billion) are sufficiently abundant that they can be put to good use to act as scientific probes—even to discover new particles.
Typically, cosmic rays, mostly protons and some heavy atomic nuclei, collide with the nuclei of nitrogen and oxygen high up in the earth's atmosphere, perhaps 10 to 20 miles up. These collisions smash the nuclei apart and send other particles as debris downward, into the atmosphere. This typically generates a plume of electrons from all of the subsequent ionization and more collisions of debris particles with other atoms. Long-term exposure to this radiation would be harmful, but we are protected by the
atmosphere at ground level. Occasionally some new particle could, in principle, be produced, and it might be detected all the way down at the surface of the earth, provided it is a deeply penetrating particle. Some experiments place detectors high up on mountaintops or in balloons to try to detect less deeply penetrating particles.
From the 1930s, and even beyond the 1950s, when particle accelerators finally became available, most of the early discoveries of new elementary particles came from cosmic ray experiments. And, to this day, cosmic rays continue to serve us well in providing information that is hard to obtain from accelerators. Most recently, the masses of neutrinos were established using cosmic rays as neutrino sources in 1995 (see
chapter 10
).
The energy stratum of the nucleus is measured in terms of millions to hundreds of millions of electron volts. To unravel the nucleus required probes of hundreds of millions of electron volts, so people in the 1930s and 1940s turned to exploit cosmic rays.
THE MYSTERY DEEPENS: WHAT HOLDS THE ATOMIC NUCLEUS TOGETHER?
By the mid-1930s, building upon Rutherford's discovery and the new quantum theory, it was realized that the atomic nucleus is composed of particles called protons and neutrons. The proton and neutron are very similar particles, each having about the same mass, but there is a big difference: the proton has a positive electric charge, while the neutron is electrically neutral. Hydrogen has the simplest nucleus that consists of a single proton, but all heavier nuclei are made of combinations of protons and neutrons, just as molecules are made of atoms. For example, the normal helium nucleus contains two protons and two neutrons.
12
Nuclei, like helium containing two or more protons, can only be held together by a very strong force—which is simply called the
strong force
. This nuclear-binding strong force has to be ultra-strong because the protons each have positive electric charges and therefore repel one another electrically. The nucleus of an atom like helium would instantly fly apart unless an overwhelmingly strong force compensated for this electrical repulsion and bound the protons, together with the neutrons, into the compact
nucleus. Indeed, a nucleus like uranium, with 92 protons, is very unstable because of the enormous repulsive electrical forces of so many protons. Uranium therefore has many
isotopes
, such as U
233
(where 233 = 92 protons + 141 neutrons), U
235
(92 protons + 143 neutrons), U
238
(92 protons + 146 neutrons), etc. Notice that we can package more neutrons into uranium because they are electrically neutral, and even help the binding together of the 92 protons. The strong force is about 10,000 times stronger than electromagnetism, and it can hold nuclei together up to about 100 protons.
Very heavy nuclei with lots of protons are generally unstable due to this electric repulsion. They undergo fission (spontaneously break apart) into lighter nuclei.
Forces, in our quantum world, are actually generated by particles. The force between two objects, like a proton and a proton, is caused by lighter particles that jump to and fro between the two protons. The repulsive electrical force is caused by the jumping of photons, the particle of light, back and forth between the protons. The strong force had to be due to something else.
A particle responsible for the strong force was predicted by the Japanese physicist Hideki Yukawa,
13
in 1935, based upon the known properties of the atomic nucleus. Yukawa reasoned that the force of electromagnetism is comparatively long range—the electric force between charged particles decreases “slowly,” by the inverse square law (it falls like 1/r
2
where r is the distance between the two particles). This inverse square law arises because the photon is a massless particle and can easily jump between nearby or distant electric charges. The force of electromagnetism is also somewhat feeble, because the “jumping probability” in quantum theory involves a small number, essentially the (square of the) electric charge (see the Appendix). This gives rise to the electric force that binds electrons to the positively charged protons in the nucleus.
On the other hand, an atomic nucleus is very small, a typical radius of about 0.0000000000001 centimeters (10
-13
cm), about one hundred thousand times smaller than the electronic orbits that define the chemical size of the atom. This arises in part because of the much larger masses of protons and neutrons than electrons, but also by the strength of the strong force that overcomes the electric repulsion between protons. Furthermore, the nucleus is quite compact, requiring that the particle of the strong force need not produce a long-range or inverse square law force (which would
have been detected outside the nucleus), but rather it is a short-range force. Yukawa realized that this required a new particle that could hop back and forth between protons and neutrons, causing the strong force, and the new particle would need to have a mass of about 100,000,000 eV (100 million eV, or 100 MeV; see
note 4
) to account for the short range of the new force.
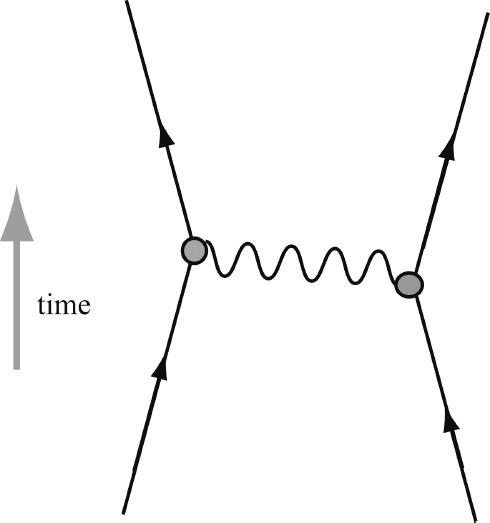
Figure 2.2. Forces Arise as the “Exchange of Particles.”
The force between two particles arises from the “exchange of particles.” Two electrons, or any electrically charged particles, interact by exchanging photons, which are the particles of light. A proton and neutron strongly interact by exchanging pions.
This is a tall order, but it certainly pointed the particle searchers in the right direction. And remarkably, in 1936 a particle with a mass of 100 MeV, called the
muon
(pronounced mew-on), or
µ
, was discovered in 1937. It seemed to be the thing predicted by Yukawa, but people soon realized that the muon was a case of false identity—the cops had arrested the wrong guy. The muon was discovered by using cosmic rays that (somehow) produced it 10 miles up in the sky. The muons then traveled to the surface of the earth where they could be detected. The reason the muon was initially thought to be the particle Yukawa had predicted (called the pion [pie-on], or
π
) was because it had Yukawa's predicted mass. But the muon did not interact strongly enough with protons and neutrons to be a pion since it could travel all the way to the earth's surface (muons only interact electromagnetically, or through the weak force). This new particle definitely was not the agent of the strong force, as predicted by Yukawa. In fact, the muon seemed to be a mere carbon copy of the electron but 200 times heavier, with a lifetime of about 2 millionths of a second (whereby the muon “decays” into an electron and a pair of neutrinos).
14