Boost Your Brain (4 page)
Authors: Majid Fotuhi

Although I’ll often refer to this part of the brain in the singular, you actually have two hippocampi, one in each hemisphere of your brain. They look identical, and both bear a passing resemblance to a sea horse. (The name comes from the Greek words
hippos
for horse and
kampos
for sea monster.) Portions of the hippocampus are tied to spatial or emotional memory, while others handle a person’s recall of a sequence of events or facts.
Your Brain’s Blueprint: CogniCity
Let’s imagine Sara’s developing brain as a collection of neighborhoods—these are the lobes you’ve just read about—connected by a complex series of highways, boulevards, and small roads. We’ll call it CogniCity. Eventually, the highways in CogniCity will be smooth and perfectly paved, all the better to whisk cars—or messages, in this case—from point A to point B. When Sara’s born, though, they’re more like dirt roads connecting small towns. Travel is neither quick nor easy.
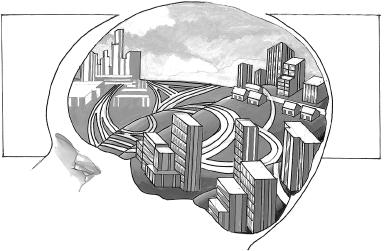
CogniCity
The brain can be thought of as a city made of neighborhoods of different sizes that are linked with each other by roads and highways.
That’s, in part, because the nerve fibers that will one day become the brain’s highways have yet to be covered with myelin, a fatty white substance that sheaths a neuron’s axon in much the same way insulation covers an electrical wire. When myelin develops—in a process called myelination—it allows brain cells to pass electrical signals effectively and efficiently. Those messages travel down the axon on their way to the synapse as a series of signals, with each hopping further down in a process called saltatory conduction. Without myelin, this saltatory conduction requires a lot more energy to be expended by the neuron. And not only do unmyelinated axons expend more energy to do their work, they also do a less-than-stellar job of it. Some signals leak out of the unmyelinated axon, never to be delivered at all.
If myelination is not completed, some parts of Sara’s brain can’t communicate well with other parts, making complex movements harder, if not impossible, to pull off.
Beyond the roads, the neighborhoods have some development ahead of them as well. They will grow, almost exclusively, through the development of dendrites and axons. The two exceptions are the cerebellum and the hippocampus. The cerebellum isn’t fully developed at birth and is one of the few parts of the brain that experience neurogenesis—the birth of new neurons—after a child is born. This happens throughout the first year of life. Incomplete development and incomplete myelination in the cerebellum mean a baby can’t yet sit up or stand and can only clumsily grasp a bottle. The hippocampus, too, is not yet fully myelinated and will also experience neurogenesis, which, as we’ll discuss throughout this book, lasts well beyond the first year and, in fact, into late life.
Still, these are the exceptions. In every other CogniCity neighborhood, future development is tied to the creation of synapses rather than the birth of new neurons.
As her brain’s highways and neighborhoods develop, Sara’s skills will begin to advance. While at birth she could only detect light, as synapses form and fiber bundles myelinate she’ll be able to differentiate patterns and color in the things she sees. Before long, Sara will be able to smile (around the two-month mark), hold her head steady (four months), and sit without support (six months). By one year, she will most likely be able to stand and take a few steps.
In the years ahead, Sara will reach yet more milestones. By age three or four, the areas of the brain responsible for memory will have begun to mature: meaning her earliest memories will stem from this age, and not before. By age six or seven, she’ll likely be able to entertain abstract thoughts. By eight or nine, she will almost certainly engage in more complex thinking.
As Sara grows, her brain will produce new connections—and cull those it doesn’t need—shaping her brain in a process that continues until about the age of twenty, or perhaps even later.
2
And It’s Done . . . or Not
By the time Sara reaches her early twenties, her brain will be considered fully developed. And yet, that doesn’t mean it’s set in stone. As you’ll soon read, Sara’s brain will still have substantial capacity to grow and change.
Unfortunately, just as CogniCity develops, it can also deteriorate. When she enters her thirties, Sara’s own CogniCity might already be showing subtle signs of decay—streetlamps flickering and flaming out, shutters hanging loose, small potholes forming in the city’s oldest roads. As she ages, Sara may find her city slowly crumbling, losing synapses through lack of use, allowing her brain’s fiber bundles to deteriorate and neurons to die from inadequate blood supply. Instead of a gleaming metropolis, her CogniCity might be a city in blight.
On MRIs, such brain aging shows up as a thinning of the cortex, which begins in middle age and continues into late life.
3
White matter fiber bundles—the network of connections between brain areas—also wither after age fifty, primarily due to reduced blood flow. The highly malleable hippocampus, meanwhile, is one of the first areas to shrink, and one of the hardest hit. It shrinks at a rate of 0.5 percent a year after the age of fifty, leading to poorer short-term memory. That’s why as she ages Sara may experience subtle memory problems long before she begins to have trouble calculating a tip, for example.
When
this all happens, and how much it affects a person, depends to a large extent on lifestyle choices. Sara wouldn’t be too unusual if she began in her fifties to experience what we call age-associated memory impairment. This means she might book a hair appointment and then forget about it until the hairdresser calls, slightly annoyed. She might blank on the name of an acquaintance. Or forget the password she set up for an online account.
Such lapses might bring on acute fears of Alzheimer’s disease for Sara, as it does for many people who have mild age-related cognitive changes. As you’ll soon read, such problems may be accelerated by lifestyle choices or certain simple medical issues, resulting in a “brain fog” that can be easily treated and reversed.
At this stage, Sara’s cognitive future would be at a bit of a crossroads: Take one path—with limited physical activity, untreated health problems, poor diet, and high stress—and she’d head toward a brain that looked like Mrs. Grey’s. But if she followed the other path—if she grew her brain—Sara would likely be headed in a very different direction.
Your Brain Late in Life
As Sara ages, some amount of cognitive decline is inevitable. Typically, such decay unfolds in a pattern that looks remarkably like development in the young brain—only in reverse.
The parts of the brain that were first to get their full share of myelination and to fully mature are the last to deteriorate. Those that developed last, on the other hand, are the first to go. To understand why and how this happens, we can look at the hippocampus, which is one of the last areas of the brain to develop and myelinate (and actually is one of the only areas of the brain to never fully myelinate). With little to no myelin insulation, neurons in the hippocampus have to work overtime—for decades—in order to pass signals to other parts of the brain. Small wonder, then, that they are the first to wear out and die. This may be the main reason why Sara will likely experience memory lapses in her fifties and sixties, long before she has difficulty with the sensation of touch or her vision (which are among the first brain areas to develop). As Sara enters her seventies and eighties, other parts of her brain will begin to shrink in this last-in, first-out progression. Eventually, as she reaches (or even passes) her nineties, the parts of her brain involved in processing speed wither away. With enough time, she might even return to the simple cognitive functioning of a child.
This whole aging process, of course, happens to varying degrees and at varying speeds, depending in part on a person’s life choices and overall health. There are, as you’ll read throughout this book, a host of health and behavioral factors that can speed up—or slow down—the brain aging process. Most often, there are multiple factors at play. Alcohol abuse, traumatic brain injury, excess stress, sleep disorders, vitamin B12 deficiency, obesity, and cardiovascular disease (among other ills) can all destroy the brain’s highways and neighborhoods.
A lifestyle with a focus on brain-boosting growth on the other hand, will help keep the brain young well into the last decade of life.
Studying the Brain
For years, much of how we tied particular actions to individual parts of the brain came from studying the brains of the dead or those with traumatic brain injuries. One of the most famous of such cases is that of a man named Phineas Gage, a then-twenty-five-year-old who in 1848 survived a railway accident in which a metal tamping rod shot through his skull. Doctors repaired the outward damage but there wasn’t much they could do for his massive brain injury.
Quiet and pleasant before the injury, Gage—who recovered physically—was said to be volatile, rude, and impulsive after being hurt, although experts today disagree on just when those symptoms began to appear and how severe they really were. Still, Gage became a case study for understanding the functions of the frontal lobes. Since it was primarily his left frontal lobe that had been damaged in the accident, the change in Gage’s behavior and demeanor offered insight into the role of the frontal lobes in decision making and impulse control.
Even more thoroughly studied than Gage was Henry Gustav Molaison, known in scientific circles as H. M. Born February 26, 1926, H. M. suffered a head injury in a bicycle accident at the age of nine. Perhaps from the accident—we don’t know for sure—H. M. began to suffer frequent epileptic seizures. The seizures were so severe that in 1953 neurosurgeon William Beecher Scoville operated on him, removing most of his hippocampus on both sides of the brain.
The surgery was successful in reducing H. M.’s seizures. But it soon became apparent that the operation had robbed H. M. of something significant: his ability to acquire new information. Without his hippocampus, H. M. was unable to commit any new information to his long-term memory. So, while he could recall much of what had happened to him before his surgery, H. M. immediately forgot anything that occurred after it.
Over the years, H. M. became something of a poster child for memory and brain research. A succession of doctors tested and prodded him, all with the hopes of understanding his curious condition. Along the way, they gleaned valuable insight into the workings of the hippocampus. The fact that H. M. remembered events and people from early in his life told doctors that long-term memories weren’t stored in the hippocampus. Similarly, the fact that he could still learn to perform a new physical task told doctors that his procedural memory was intact, and that this type of memory, therefore, probably wasn’t stored in the hippocampus. His inability to recall new information, too, pointed to the hippocampus having a role in the making of new memories.
H. M. died in 2008. His brain, long a source of scientific interest, is still under study. In fact, at the Brain Observatory at University of California, San Diego, Project HM researchers are currently slicing into H. M.’s brain to see what new information it may yield.
Right about the time that H. M. went under the knife, another surgeon was compiling a treasure trove of data he’d collected while performing brain surgery. Dr. Wilder Penfield, a neurosurgeon at the Montreal Neurological Institute in Canada, had performed groundbreaking brain surgeries on epileptic patients throughout the 1940s. The surgeries involved deliberately killing neurons in an effort to reduce epileptic seizures. In order to determine which cells to destroy, Penfield delivered a small electrical shock to parts of the brain and then recorded the body’s response. Patients were awake during the surgery—Penfield used local anesthesia—so depending on where the shock was delivered they might exhibit a twitch in the arm, for example, or suddenly recall a childhood memory. The technique served its intended purpose, helping Penfield determine which cells to target. But Penfield’s records on how patients responded to shocks to various parts of the brain also allowed him to expand on the limited map of brain function that existed at the time.
As the field of neuroscience developed and grew, so too did the map. Studies on animals helped pave the way, as has the development of a variety of non-invasive technologies for humans, such as magnetic resonance imaging (MRI), functional magnetic resonance imaging (fMRI), electroencephalography (EEG), and positron-emission tomography (PET), among other tools. Such high-tech tools have allowed neuroscientists to peek beneath the human skull to pinpoint just which brain parts control which functions.
Today, advances in scanning technologies have continued to expand our ability to watch the brain in action, even right down to individual groups of nerve cells. Of course, while we’ve made mind-boggling progress in understanding how the brain works, there’s still plenty of mystery left.