Brilliant Blunders: From Darwin to Einstein - Colossal Mistakes by Great Scientists That Changed Our Understanding of Life and the Universe (16 page)
Authors: Mario Livio

In the fields of observation, chance favors only the mind that is prepared.
—LOUIS PASTEUR
T
he lecture hall in the Kerckhoff Laboratory building at Caltech had rarely been as packed as it was that day in December 1950. Rumor had it that the famous chemist Linus Pauling was about to reveal something truly dramatic—maybe even a solution to one of life’s greatest mysteries. When Pauling finally arrived, one of his research assistants was carrying an object that looked like a large sculpture, covered by a piece of cloth fastened with a string. The lecture itself demonstrated yet again Pauling’s virtuosic command of chemistry, coupled with his exquisite showmanship. After keeping his audience in suspense for a while, Pauling finally used his jackknife to cut the string and, like a magician producing a rabbit out of a hat, unveiled what has become known as the
alpha-helix
: a three-dimensional stick-and-ball model of the main structural feature of many proteins.
One of the people who soon after heard about Pauling’s pyrotechnical talk, even though at the time he was thousands of miles away in Geneva, Switzerland, was James Watson, who only three years later would discover (with Francis Crick) the structure of DNA.
Watson was visiting the Swiss molecular biologist Jean
Weigle, who happened to be just back from spending a winter at Caltech. Even though Weigle could not quite judge the correctness of Pauling’s multicolored wooden model, his report on the dazzling lecture was sufficient to intrigue and embolden Watson. We shall return to that gripping story later in the chapter.
By September 1951, the account of Pauling’s scientific achievement
had made it even into the pages of
Life
magazine, where a photograph of a grinning Pauling pointing to his alpha-helix model was accompanied by the headline “Chemists Solve a Great Mystery: Protein Structure Is Determined.” The
Life
article was but a brief summary, in lay terms, of what had been a truly miraculous year in Pauling’s long career. Suffice it to note that the May 1951 issue of the
Proceedings of the National Academy of Sciences
contained no fewer than seven papers by Pauling and his collaborator, chemist Robert Corey, on the topic of the structure of proteins ranging from collagen (the most abundant protein in mammals) to the shafts of feathers. This publication marked the culmination of fifteen years of trailblazing research by Pauling.
The Road to the Alpha-Helix
Pauling started to think about proteins in the 1930s.
His first papers on the subject proposed a theory for hemoglobin—the iron-containing protein in red blood cells—suggesting that each of the four iron atoms in the molecule formed a chemical bond with an oxygen molecule. While working on that subject, Pauling pioneered a new experimental technique. He came up with the idea that measuring the magnetic properties of some proteins could provide important information on the nature of the bonds formed by iron atoms with the groups surrounding them. The method has indeed proved to be a fruitful tool in structural chemistry. Pauling used the magnetic characteristics to good effect; for instance, to determine the rates of several chemical reactions.
Around the same time,
Alfred Mirsky, a leading protein expert, came to Pasadena for a year to work with Pauling’s group. This
chance collaboration between the two scientists became the starting point for an immensely successful quest.
Mirsky and Pauling first proposed that a native protein—that is, an unaltered protein in its natural state inside the cell—
is composed of chains of amino acids known as
polypeptides,
which are folded in some regular fashion. Very soon thereafter, Pauling realized that a key question was the precise nature of this folding. Fortunately, a few clues were starting to emerge in the early 1930s from X-ray diffraction experiments. In this powerful technique, scientists shine an X-ray beam onto a crystal. Then they can attempt to reconstruct the structure of the crystal (in terms of distances between atoms and their mutual orientations) from the way the invisible rays bounce off the sample. Pauling had at his disposal X-ray diffraction patterns
obtained by the physicist William Astbury from hair, wool, horns, and fingernails (proteins known as
alpha keratin
). The X-ray photographs were rather fuzzy, however, and they did not allow for reliable structure determinations. Nevertheless, the photos did appear to indicate that the structural unit was repeating along the hair’s axis every 5.1 angstroms. (One angstrom is a unit of length equal to one hundred-millionth of a centimeter.) Given the relatively poor quality of the X-ray patterns, Pauling decided to attack the problem from the other end: to use structural chemistry—the expected interactions among atoms—to predict the dimensions and shape of the polypeptide chain, and then to check which one of the various potential configurations was consistent with the information deduced from the X-ray images.
Pauling immersed himself in the work on the folding riddle in the early summer of 1937, when he was finally free of his teaching duties.
Figure 11 shows a schematic drawing of the type of general structure that he was considering. By scrutinizing carefully the chemical bond between the carbon atom (denoted by “C” in the figure) and its adjacent nitrogen atom (denoted by “N”), Pauling concluded that the carbon, nitrogen, and the four neighboring atoms (collectively known as the peptide group) had to lie
in the same plane
. This particular feature turned out to be extremely important because it restricted greatly the number of possible structures, and Pauling therefore hoped to be able to pin down the correct configuration. Science, however, rarely proceeds precisely as expected. In spite of several weeks of very intensive work, Pauling was unable to find a way of folding the peptide chains that would reproduce the repeat every 5.1 angstroms along the fiber axis that the X-ray results seemed to indicate. Frustrated, he gave up at that point.
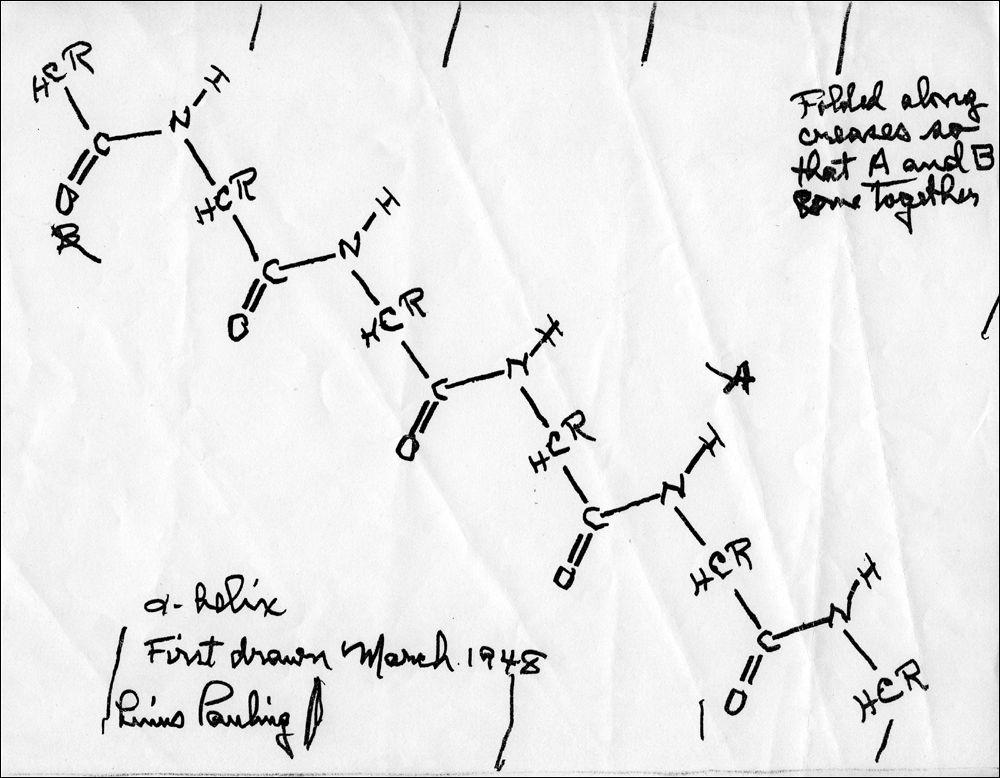
Figure 11
When a promising hypothesis doesn’t quite work, scientists often attempt to improve the quality of the available experimental data, since superior information can reveal previously indiscernible pointers. In this spirit,
Pauling convinced Robert Corey to embark on a long-term project intended to determine the structure of some simple peptides and amino acids—the building blocks of proteins—using X-ray crystallography. Corey plunged into this study wholeheartedly, and by 1948, he and his collaborators at Caltech managed to unearth the exact architecture of about a dozen such compounds. Realizing that all of Corey’s findings about the chemical bond lengths and the angles between different parts of the molecules, as
well as the
planarity
(the atoms lying in the same plane) of the peptide group, agreed precisely with his own previous formulations, Pauling decided to revisit the problem of the structure of the alpha keratin protein. In an account dictated on his (by then ancient) Dictaphone in 1982, Pauling recalled the circumstances:
In the spring of 1948, I was in Oxford, England, serving as George Eastman Professor for the year and as a fellow of Balliol College. I caught cold and was required to stay in bed for about three days. After two days I had got tired of reading detective stories and science fiction, and I began thinking about the structure of proteins.
Pauling started his new onslaught on the puzzler with the assumption that all the amino acids in the alpha keratin should be in a structurally similar position with respect to the polypeptide chain. While still in bed, he asked his wife, Ava Helen, to bring him a pencil, a ruler, and a piece of paper. Keeping each peptide group in the plane of the paper, using heavier and lighter lines to indicate the three-dimensional relationships, and rotating around the two single bonds to the carbon atoms (with the angle of rotation being the same from one peptide group to the next),
Pauling created a
helix
, a spiral-staircase-like structure, in which the polypeptide backbone formed the core of the helix, and the amino acids projected outward (
figure 12
). To stabilize the construction, Pauling formed hydrogen bonds between one turn of the helix and the next turn, parallel to the helix axis. (
Figure 12
; a hydrogen bond is a chemical bond in which a hydrogen atom of one molecule is attracted to an atom of another molecule.) He actually found two structures that could work, one of which he called the alpha-helix, and the other the gamma-helix. That Pauling was able to find solutions to the problem with such primitive
tools attests to how crucial his previous discovery of the planarity of the peptide group had been. (Figure 11 represents his attempt to reconstruct the original piece of paper from 1948.) Without it, the number of possible conformations would have been much larger. Excited, Pauling asked his wife to bring him a slide rule (long obsolete, this was the most commonly used calculation tool at the time), so that he could calculate the repeat distance along the fiber axis. He discovered that the structure of the alpha-helix was repeating after 18 amino acids in five turns. That is, the alpha-helix had 3.6 amino acids per turn. Alas, to his disappointment, the calculated distance between turns was 5.4 angstroms, and not the 5.1 angstroms hinted at by the X-ray diffraction patterns. The gamma-helix had a hole down its center that was too small to be occupied by other molecules, so Pauling concentrated his attention on the alpha-helix. Feeling fairly confident in the correctness of his solution, Pauling tried very hard to find some way to adjust either the bond lengths or the bond angles so as to decrease the calculated distance from 5.4 to 5.1 angstroms, but he failed to do so. Consequently, even though he was extremely pleased with his alpha-helix, he decided to refrain from publishing the model until he could understand better the reason for the discrepancy in the spacing.
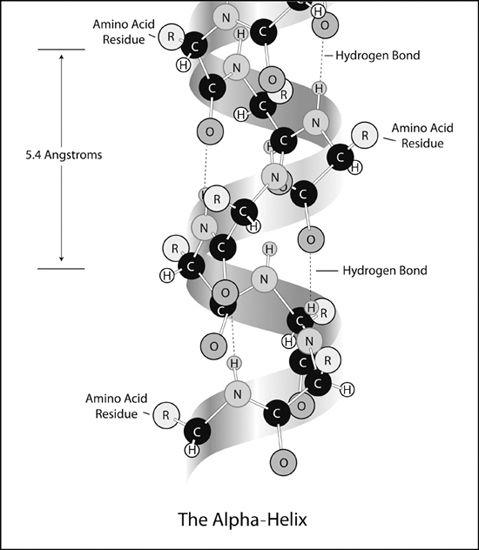
Figure 12
About six weeks later, Pauling visited the Cavendish Laboratory at Cambridge, and what he saw there impressed him deeply.
“They have about five times as great an outfit as ours,” he wrote to his assistant at Caltech, “with facilities for taking nearly 30 X-ray pictures at the same time.” Concerned that there was something still wrong with his model, and at the same time anxious that the Cavendish group might beat him to analyzing it, Pauling remained silent about the alpha-helix.
Even during a discussion with the famous chemist Max Perutz, in which the latter showed him exciting new results on the structure of the hemoglobin crystal, Pauling decided to keep his ideas to himself.
The problem, however, continued to haunt him. Upon his return to Pasadena, Pauling immediately asked a visiting professor of physics, Herman Branson, to inspect his calculations carefully. Pauling was particularly interested to know
whether Branson could find a third helical structure that would satisfy the restrictions of a planar peptide bond and maximum hydrogen bonding for stability. Branson and one of Pauling’s research assistants, Sidney Weinbaum, went over Pauling’s computations with a fine-tooth comb for about a year and concluded that there were truly only two structures—the alpha-helix and the gamma-helix—that satisfied all the constraints. Branson and Weinbaum also confirmed that the alpha-helix, which was the tighter of the two helices, was characterized by a distance of 5.4 angstroms between turns.
Pauling was now presented with the choice of whether simply to ignore the incongruity with the X-ray data and to publish his model or to delay publication until that conundrum was resolved fully. A paper submitted for publication to the
Proceedings of the Royal Society of London
on March 31, 1950, helped him decide.
I Wish I Had Made You Angry Earlier