I Can Hear You Whisper (13 page)
Read I Can Hear You Whisper Online
Authors: Lydia Denworth

The message of the emerging deaf civil rights movement was this: Deafness is not a disability; it is a difference. It is
not something to be “cured” or “fixed.” It is even something of which a person can be proud. The new consciousness found its greatest, most powerful expression in the
Deaf President Now protest at Gallaudet University in March 1988.
 â¢Â â¢Â â¢Â
WE STILL HAVE A DREAM
! read the banner. It was so big it took up most of a city street and required more than thirty people to carry it. In a nice bit of historical solidarity, the banner had been borrowed from the Crispus Attucks Museum in Washington, DC, and had last been used to rally for a national holiday in honor of Martin Luther King Jr. But this time, on March 11, 1988, it was carried by deaf students from Gallaudet University. Although the rounded dome of the Capitol Building is visible from the upper floors of buildings on the Gallaudet campus, the students were borrowing another tradition from previous civil rights protests and marching on the Capitol as the culmination of a week of tumult and emotion. They were now carrying their concerns beyond their own community and out to the wider world.
All week, the students had demanded that Gallaudet's board of trustees appoint a deaf president. In its 124 years of existence, that was something the university had never had. Over time, more and more deaf administrators and faculty had been appointed, but the president and most of the board members, including the chair, Jane Bassett Spilman, were hearing. What's more, Spilman and most of the hearing trustees had never learned to sign. In the eyes of the Deaf community, that lumped them squarely in the paternalistic tradition of hearing people who think they know what's right for deaf people without having any idea what being deaf is all about. The notion that deaf people were not capable of managing their own university was deeply offensive.
When the previous president, Jerry Lee, stepped down in 1987 and a search committee was formed, members of the Deaf community had made clear their preference for a deaf president. By early 1988, they were sure it was their moment. “It's time!” read an invitation to show solidarity at a rally on March 1. “In 1842, a Roman Catholic became president of the University of Notre Dame. In 1875, a woman became president of Wellesley College. In 1886, a Jew became president of Yeshiva University. In 1926, a black person became president of Howard University. AND in 1988, the Gallaudet University presidency belongs to a DEAF person.”
But on Sunday night, March 6, the trustees announced in a press release that Elisabeth Ann Zinser, the only remaining hearing candidate, vice-chancellor of the University of North Carolina at Greensboro, would be the next president. Shock, anger, and disbelief raged through campus. An angry crowd of students, faculty, and alumni set fire to copies of the press release and then, gathering outside Gallaudet's main gate on Florida Avenue, they decided to march into the center of Washington to the Mayflower Renaissance hotel, where the board meeting had been held, to demand an explanation from Spilman.
When she finally met with the student leaders that night, they understood her to say, “Deaf people are not ready to function in the hearing world.” She later maintained that she had not said that and never would have, and that her interpreter had misunderstood her. But the damage was done. The statement fueled a fire of frustration and resentment that had been smoldering for years. A few days later, Spilman again embodied the problem when she stood at the podium at Gallaudet's field house as angry, jeering students refused to pay attention.
“We will not sit here if you are going to scream so loudly that we cannot hear you and that we cannot establish a dialogue,” said Spilman.
As her words were interpreted, the students replied that there was no noise for them. Later they set off the building's fire alarms, just for good measure.
By the end of the week, the demonstrations had succeeded completely. Elisabeth Zinser resigned before she ever set foot on the campus, and the board appointed I. King Jordan, a deaf psychology professor, as the new president.
The protest had been heard far beyond the gates of the university and the meeting rooms of the Mayflower hotel. On March 11, the day of the march on the Capitol, ABC News named the Gallaudet student government president, Greg Hlibok, as its Person of the Week.
“This week, millions of us have had the chance to sit forward in our chairs and watch a group of young people as they break down some very out-of-date stereotypes,” said ABC's anchor Peter Jennings in his introduction.
Then Hlibok, a slim, clean-cut young man in a jacket and tie, appeared. “Deaf people are capable of anything except that they can't hear,” he said through an interpreter.
Jennings recounted the events of the week, the “test of wills” that had brought down a university president and raised the national consciousness for hearing people. For deaf people, he said, it amounted to “a national catharsis.”
“It seemed as if there'd never been such an opportunity,” said Jennings, “to tell the world that deaf does not mean defeated.” For the first time ever, it was hearing people who had come away from a collision dented.
Into the turbulence of nascent deaf civil rights dropped the cochlear implant.
L
ANGUAGE IN THE
B
RAIN
I
t was about eight months after Alex got his hearing aids and started at Clarke, and Mark and I were sitting anxiously in an exam room at New York Eye and Ear Infirmary while Alex, now two and a half, played with the pale green vinyl footrest of the exam chair. We were waiting for Dr. Simon Parisier. Medically speaking, we had graduated to the big leagues.
“We need to implant him,” Dr. Parisier said soon after he came in.
A few months earlier, at the beginning of September, Alex and I had returned to Jessica O'Gara for what we thought was a routine appointment to fine-tune the settings on his hearing aids. We got an unwelcome surprise: All of the hearing in his right ear was gone. He was now profoundly deaf on that side. Through the summer, we had watched obsessively for bumps on the head that might worsen his hearing, but nothing had happened. Or so it had seemed. Perhaps, as Dr. Dolitsky had warned, Alex's remaining hearing had been lost from something as seemingly minor as a sneeze. If the right ear had dropped so precipitously, it was likely only a matter of time before the left ear followed. Then came a speech and language evaluation at the beginning of Decemberâa year after the first one. Alex had moved from the second to the sixth percentile for receptive language and was still in the eighth percentile for expressive language.
“Single digits? Still?” I gasped when I got the results. “But he has more than two hundred words now! That can't be right.”
It was right. Alex had been working hard and learning every day. But so was every other two-year-old. No one was going to stand still and wait for him. That is why achievement gaps are so stubbornly hard to close. The learning curve for children who start behind can't simply parallel that of typical children. To catch up, their progress requires the steep trajectory of a rocket. Alex's new drop in hearing was at least a reasonable explanation for why he was moving slowly. His right hearing aid was no longer providing useful sound. He was down to one ear, and a limited one at that.
Immediately, Dr. Dolitsky had sent us to see Dr. Parisier, one of the pioneers of cochlear implant surgery. As a younger doctor in the 1970s at Mount Sinai Hospital in New York City, Parisier had embraced the potential of the new technology from the start. Back when he graduated from medical school in 1961, the introduction of antibiotics had just radically changed the field of ear surgery. Before that, he says, “it wasn't uncommon to die from an ear infection. In the 1950s, doctors hadn't been concerned about hearing, but with saving lives. There was nothing you could do about nerve deafness.” With the introduction of implants, Parisier felt there might be something he could do to help previously unhelpable patients. He found meningitis survivors particularly compelling. “Meningitis was the single largest cause of deafness in kids,” he told me. “Ten percent of those who survive become profoundly deaf. It was very painful. A child goes into the hospital able to speak at two or three years old and within days, that speech goes. When they wake up, they don't talk anymore.” Even six- and seven-year-olds struggled and lost their language. The experience taught Parisier about the importance of getting language early. It worried him that Alex was already two and a half.
A new round of tests ensued, this time to see if Alex was a candidate for a cochlear implant. Mark and I began to investigate what this would mean. At Clarke, which serves children through age five at the New York campus, I already knew many kids with implants. Some spoke intelligibly, some much less so. I knew the device didn't bring instantaneous success, but also that Alex would be starting with the advantage of having some hearing in the other ear.
We brought home videotapes prepared by the three cochlear implant manufacturers. Of course, they showcased the success stories. But what stories they were. In the “before” videos, the children were Alex's age, laboring with speech therapists to pronounce a few words. In the “after” videos, they were school-age, in mainstream classrooms, all of them speaking, some of them virtually indistinguishable from their hearing classmates. One particularly captivating profoundly deaf girl had used sign language until she was three, when she got her implant. By the movie's end, she was nine, a busy, happy fourth-grader, singing in her mainstream school's choir. As the credits on the video ran, Mark and I looked at each other.
“That's what I want,” he said.
“Me too,” I agreed.
We emerged with a case of emotional whiplash: We went from desperately wanting to hang on to every decibel of Alex's hearing to desperately hoping his hearing was bad enough to qualify him as a candidate.
White-haired and soft-spoken, Dr. Parisier had a gentle, matter-of-fact manner, but he didn't mince words when he delivered his opinion. He clipped several CT scan images of Alex's head up on the light board and tapped a file containing the reports of Alex's latest hearing tests and speech/language evaluations. “He is not getting what he needs from the hearing aids. There's no high-frequency access. His language is not developing the way we'd like,” he said. Then he turned and looked directly at us. “We should implant him before he turns three.”
A deadline? So there was now a countdown clock to spoken language ticking away in Alex's head? What would happen when it reached zero? Alex's third birthday was only a few months away.
As Parisier explained that the age of three marked a critical juncture in the development of language, I began to truly understand that we were not just talking about Alex's ears. We were talking about his brain.
 â¢Â â¢Â â¢Â
Even more than his hearing, Alex's brain was mysterious. It required faith. I couldn't touch it or caress it, as I did his ears, running my fingertips along their small curving lobes. Of course, I knew his brain was there. When he breathed or blinked or blew me a kiss, it was sending proof of its existence, like postcards from a far-off land I can point to on the globe but never visit. Still, I struggled to imagine the reality of it. Even the vocabulary of the brain is hard to fathom with its map of place names that sound simultaneously alien and ancientâsuperior temporal gyrus and perisylvian cortex, amgydala and hippocampus.
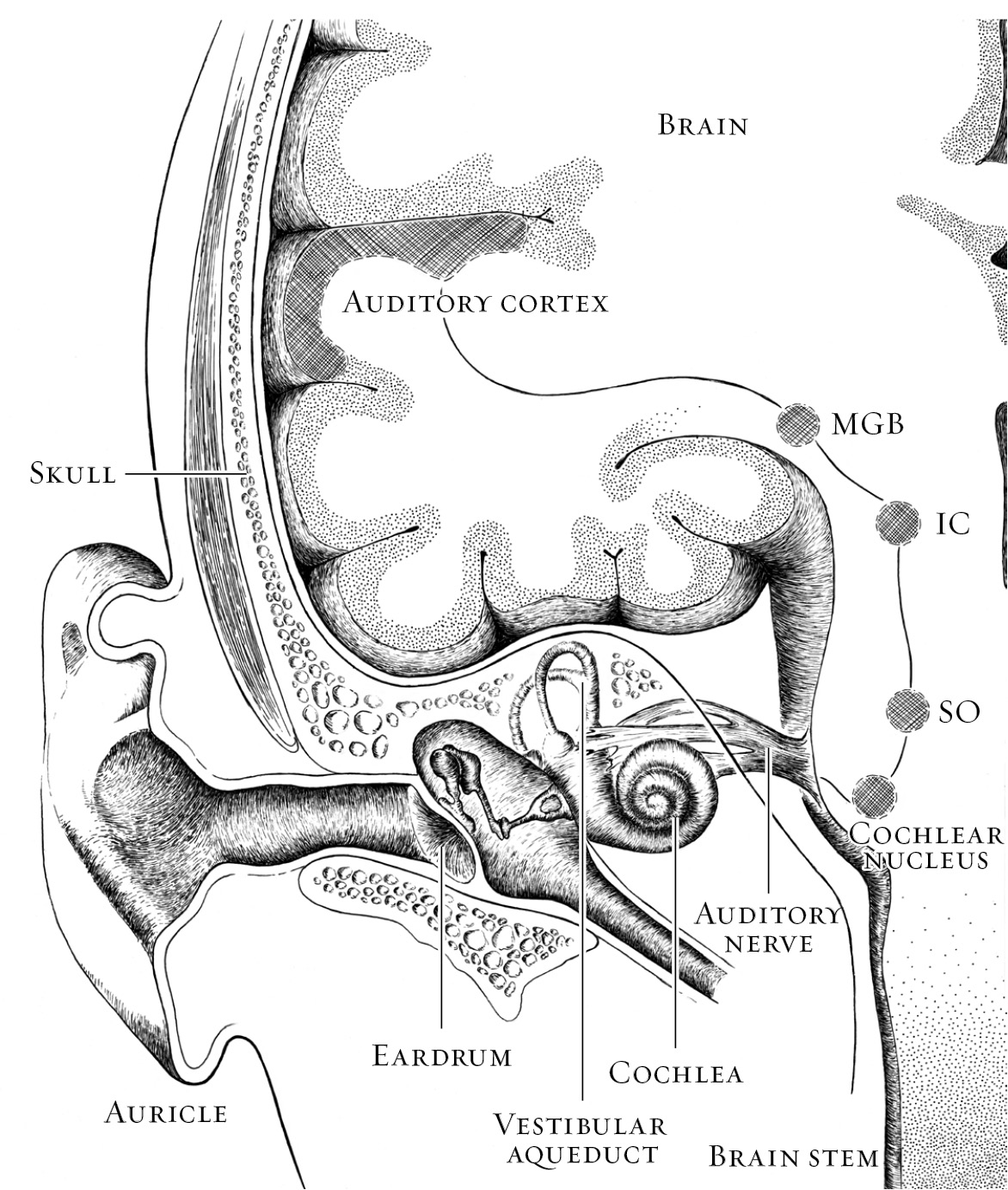
In among the models and diagrams of the ear in every doctor's office we had visited, there was not one map of the brain on the wall. Perhaps there should have been. I wanted to know more than that the cochlear implant would deliver sound to Alex's auditory nerve, which would convey it to the brain. I needed to know what would happen to that sound once it arrived. I needed to know what the brain would do with that sound while Alex was two that it might not be able to do once he turned three.
The answers lay in the burgeoning science of brain plasticityâthe study of the capacity of the brain to change with experience. Helped by advances in technology, neuroscientists have built up atlases of normal brain development. That is the starting point for understanding what happens in brains that are atypical.
The differences between a brain at birth and that same brain at twenty-one years of age are considerable and driven both by genetics, which is “
merely an opening gambit on nature's part,” as science writer Sharon Begley put it, and by environment. The baby's brain arrives in the world primed for learning. It is now thought to contain seventy to eighty billion neurons, or nerve cells, down from previous estimates of one hundred billion, but still most of the neurons a person will need as an adult. What is lacking is communication from neuron to neuron. The process of generating the remaining necessary neurons and creating connections between neurons is what the learning of childhood is all about.
Neurons are often compared to trees, with roots and branches, but to me they look more like spindly sea creatures or insects with long skinny bodies and arms like tendrils waving away at each end. Information comes in through those tendrils, the dendrites, and travels along the axon to the other end of the cell. At the base of each axon there's a gap before the next neuron. The electrical signal is carried over that gap, or synapse, by chemical neurotransmitters, like a written message wrapped around the shaft of an arrow and fired over a moat.
The warmth of a baby blanket, the smell of a father's aftershave, the sight of a mother's loving gaze, the soothing melody of a lullaby: Together, these perceptions contribute to a baby's sense of security, but each is also altering the configuration of the baby's brain. Each is a piece of sensory information that travels through the nervous system to the appropriate area of the brain. The image of a mother's face, for example, enters the retina of the eyes and passes along a pathway to the visual cortex in the occipital lobe at the back of the brain. Every time the baby sees his mother's face, the same chain of nerve cells fires. As neuroscientists say, neurons that fire together wire together. Pretty soon, a circuit has been created and the baby recognizes his mother instantly. There are similarly specialized areas for touch, sound, and smell.
In this manner, information travels through the brain leaping from neuron to neuron, creating circuits that receive and then process and act on information. Zip, zip, zip. The more often a specific signal is sent down a particular path, the more defined and efficient that path becomes, like a hiking trail that is well maintained versus one that's overgrown. Paths that are traveled less often or not at allâconnections that aren't made, in other wordsâeventually disappear. The brain assumes they are unnecessary and prunes them away. Snip, snip, snip. As neuroscientist Helen Neville puts it, “
experience is like a sculptor who begins with more clay than he needs.” This idea is somewhat counterintuitiveâthat a child's brain is simultaneously strengthening and eliminating brain circuits. But that is the beauty of the system. It is as if the landscape of the brain is under the control of a master gardener who creates paths where they are wanted, plants new seeds as necessary, directs the flowerings and branchings, and cuts back to concentrate growth where it is most desirable.
Today, scientists can watch the brain maturation process unfold. In a prospective study published in 2004, a group of researchers based at the
National Institutes of Health and UCLA followed thirteen healthy children for close to ten years. The children, who ranged in age throughout the study from four to twenty-one, underwent functional magnetic resonance imaging (fMRI) every two years. The images were color-coded to indicate relative levels of gray matter density in the children's brains, with red and yellow indicating more gray matter and blue and purple less. As neural connections explode early in childhood, gray matter density increases. As those connections are pruned back and insulated with myelin, that gray matter volume decreases and white matter increases. So more white matter indicates a more mature brain.
In the time-lapse movie of the results, large swathes of yellow and red are slowly washed with blue as the exuberant bursts of neuron growth in early childhood become cooler and more efficient, mature neural pathways. The sequences showed that the brain's most basic functions, those concerning vision and touch, developed first, followed by centers for language in late childhood and early adolescence and then, toward early adulthood, by the areas that control reasoning, self-reflection, and planning. (Interestingly, in Alzheimer's patients the sequence is reversed. Their brain matter loss progresses from front to back, beginning with the higher-order functioning and finally enveloping more basic functions of the brain.)
Most of the growth in neurons takes place between birth and the age of five, which is why the UCLA movies, whose youngest subjects were four, begin with brains full of gray matter (i.e., uninsulated neurons). The pruning begins in earnest around five, and children will lose 35 percent of the nerve cells they have at five before adulthood. For many years, the assumption was that after early childhood our brains did not continue to generate new nerve cells, but within the past ten years, researchers discovered
a second, smaller wave of neuron creation in puberty. And we now know that it is
possible to keep learningâand even generating new neuronsâinto adulthood, though it takes far more effort to see results.
According to the brain's basic division of labor, sensory areas receive all the incoming information from our eyes, ears, nostrils, tongue, and fingertips. The back of the brain, the occipital lobe, handles vision. Hearing is processed in the temporal lobe on the side of the head just above the ear. A strip across the top of the head collects information about everything we touch, whether with our toes, palms, shoulder, cheek, or any other part of the body. Motor areas control our movements. The limbic system is involved with emotion. “Cortex” is the collective name for the exterior surface of the brain, the wavy, lumpy gray matter familiar from anatomy books. It's here, particularly in the section right behind the forehead known as the prefrontal cortex, that the higher-order processing occurs that marks us as distinctly human and controls our ability to plan, to reason, to remember, to reflect.
The central auditory system begins where the inner ear passes a signal to the auditory nerve. A part of the eighth cranial nerve, the auditory nerve is not just one nerve but a bundle of nerve fibersâa coaxial cable of sortsâconnecting the cochlea to the brain stem. Within the brain stem, where the auditory nerve ends at two collections of neurons called the cochlear nuclei, things start to get complicated. That is as it should be. Very literally, brain processing gets more sophisticated as it ascends, the way a good curriculum builds on itself and asks more of students as they move through school.