Life's Greatest Secret (28 page)
Read Life's Greatest Secret Online
Authors: Matthew Cobb

I find it impossible to form any considered judgement of this idea. It may be complete nonsense, or it may be the heart of the matter. Only time will show.
20
Time showed it to be complete nonsense. More importantly, the idea may have restricted what researchers thought was possible, in particular because it ruled out triplets composed of the same base. For most people trying to crack the code, investigating the effect of a polynucleotide containing only one kind of base would have been pointless.
*
By the end of 1960, Nirenberg and Matthaei were working through the night on protein synthesis in
E. coli
extracts. In mid-January, one of Nirenberg’s diary entries was headed
‘Idea. Approach to code’
and outlined the use of poly(A), poly(U), poly(C) or poly(G) and of poly(AG), etc.; poly(AG) would be composed of equal amounts of A and G, in an unknown sequence. Nirenberg’s aim was to put polynucleotides into his cell-free protein synthesis system and use the output to understand the nature of the genetic code, starting with establishing the number of bases involved in coding for an amino acid:
E. coli
extracts. In mid-January, one of Nirenberg’s diary entries was headed
‘Idea. Approach to code’
and outlined the use of poly(A), poly(U), poly(C) or poly(G) and of poly(AG), etc.; poly(AG) would be composed of equal amounts of A and G, in an unknown sequence. Nirenberg’s aim was to put polynucleotides into his cell-free protein synthesis system and use the output to understand the nature of the genetic code, starting with establishing the number of bases involved in coding for an amino acid:
Might be able to get enough info to establish limits of a code … If you need all 4 bases, could not be triplet code.
21
At the FASEB Meeting in February 1961, Matthaei and Nirenberg gave a brief talk describing the way their system incorporated a
14
C-labelled amino acid (valine) into a protein.
22
A few weeks later, on 22 March, they submitted an article on the topic to
Biochemical and Biophysical Research Communications
.
23
This journal had been set up in the previous year to respond to the increasingly fierce competition in the area by offering rapid publication of short articles – it used camera-ready typed copy provided by the authors rather than traditional typesetting, thereby speeding up the whole publishing process.
14
C-labelled amino acid (valine) into a protein.
22
A few weeks later, on 22 March, they submitted an article on the topic to
Biochemical and Biophysical Research Communications
.
23
This journal had been set up in the previous year to respond to the increasingly fierce competition in the area by offering rapid publication of short articles – it used camera-ready typed copy provided by the authors rather than traditional typesetting, thereby speeding up the whole publishing process.
The article described how the presence of ribosomal RNA in their cell-free system was essential for amino acid incorporation, which ‘had many characteristics expected of protein synthesis’, and that ‘all of the activity appeared to be associated with RNA’.
24
Exactly what kind of RNA Nirenberg and Matthaei were referring to was not entirely clear. They concluded, somewhat confusingly ‘It is possible that part or all of the ribosomal RNA used in our study corresponds to template or messenger RNA.’
25
By ribosomal RNA they did not mean the RNA that makes up the ribosome itself, but rather an RNA molecule that was attached to the ribosome. The ambiguity contained in the term ‘template or messenger RNA’ is not simply a matter of uncertainty over which word to choose. As Lily Kay has pointed out, Nirenberg and Matthaei’s use of ‘template or messenger RNA’ shows that their language was poised at a cusp between the old, physical, way of thinking of specificity – as a structural template – and the new, abstract idea of information being transferred by a messenger.
26
Understandably, these semiotic niceties were not noticed at the time, and the paper made no impression – it was not cited until 1963, by which time all the dust had settled.
24
Exactly what kind of RNA Nirenberg and Matthaei were referring to was not entirely clear. They concluded, somewhat confusingly ‘It is possible that part or all of the ribosomal RNA used in our study corresponds to template or messenger RNA.’
25
By ribosomal RNA they did not mean the RNA that makes up the ribosome itself, but rather an RNA molecule that was attached to the ribosome. The ambiguity contained in the term ‘template or messenger RNA’ is not simply a matter of uncertainty over which word to choose. As Lily Kay has pointed out, Nirenberg and Matthaei’s use of ‘template or messenger RNA’ shows that their language was poised at a cusp between the old, physical, way of thinking of specificity – as a structural template – and the new, abstract idea of information being transferred by a messenger.
26
Understandably, these semiotic niceties were not noticed at the time, and the paper made no impression – it was not cited until 1963, by which time all the dust had settled.
In early May 1961, Nirenberg and Matthaei decided to add RNA from the tobacco mosaic virus (TMV) to see if they could get the cell-free system to synthesise TMV protein. It worked like a dream. As Nirenberg recalled in the 1970s, the results were
‘superb … beautiful
… It was superbly active’.
27
He realised that they would need to collaborate with the Berkeley TMV expert Fraenkel-Conrat if they were to fully exploit this novel approach. In the meantime, they continued to crank through the effects of the various synthetic RNA molecules that they were able to borrow from Leon Heppel’s laboratory next door.
‘superb … beautiful
… It was superbly active’.
27
He realised that they would need to collaborate with the Berkeley TMV expert Fraenkel-Conrat if they were to fully exploit this novel approach. In the meantime, they continued to crank through the effects of the various synthetic RNA molecules that they were able to borrow from Leon Heppel’s laboratory next door.
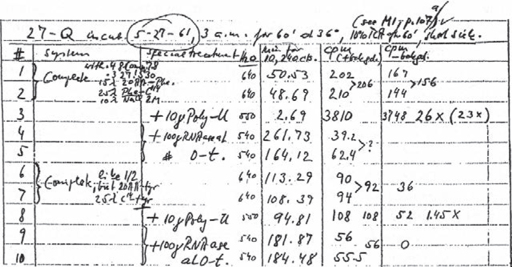
9. Matthaei’s notebook showing the results of the crucial experiment – reproduced from Kay (2000)
In the middle of May, Nirenberg left to spend a month in Fraenkel-Conrat’s lab in Berkeley, getting himself up to speed with TMV. Back in Bethesda, Matthaei started a set of experiments to study the response of the cell-free system when it was seeded with artificial RNAs. On 15 May (his 32nd birthday), Matthaei began an experiment testing the effect of poly(A) (AAAAA…), poly(U) (UUUUU…), poly(2A)U (a ratio of two A nucleotides to one U, randomly distributed through the RNA molecule), and poly(4A)U (a randomly distributed ratio of four A nucleotides to one U). When all twenty amino acids added to the test tube were radioactive, Matthaei obtained a twelvefold increase in radioactivity in the protein product after incubation with poly(U), a small increase with poly(AU), and barely any change with poly(A). Something was going on in the poly(U) tube, which could explain how genetic information leads to a particular protein being created. To detect which radioactive amino acid had been incorporated into the protein that was produced by the cell-free set-up, Matthaei had to test all twenty amino acids systematically. He did this by putting ten radioactive amino acids into a test tube – the other ten amino acids added were the usual ‘cold’ versions. He then did the poly(U) experiment again. If there was an increase in radioactivity, then clearly one of the ‘hot’ amino acids was involved. By repeating this process, Matthaei was finally able to narrow the effect down to one of two amino acids: phenylalanine or tyrosine.
On Saturday 27 May at 3.00 a.m., Matthaei began the final experiment. This involved ten test tubes and was labelled ’27-Q’ in his lab book. In tube number 3 he had nineteen unlabelled amino acids together with radioactive phenylalanine, and in tube number 8 he had nineteen unlabelled amino acids together with radioactive tyrosine. The remaining eight tubes contained various controls to prove that the effect was due to the combination of the poly(U) and one of the two radioactive amino acids. Matthaei allowed the mixture to incubate for one hour at 36°C; then he began the tedious task of isolating the protein produced by the reactions and measuring the radioactivity that had been incorporated. Tube 3, which contained radioactive phenylalanine, produced a protein with a radioactivity level that was more than twenty times higher than the control tubes; the protein from the tyrosine tube showed no increased radioactivity. When Gordon Tomkins came into the lab a few hours later, Matthaei told him the news: poly(U) coded for phenylalanine. The first word in the genetic code had been read.
*
Nirenberg, who was at Berkeley, heard of the breakthrough over the telephone, and by 11 June he was back at Bethesda doing experiments. Matthaei recalled the feelings in the laboratory at the time: ‘of course we were excited, because we knew
exactly
what we had. And we knew what we’d wanted to get.’
28
Everyone was sworn to secrecy – nobody was to hear about the finding until the results had been published. This caused some difficulties – at the beginning of June, Sydney Brenner gave a talk at Bethesda, during which he said it was not possible to study messenger RNA in a cell-free system. When Matthaei asked him how he could know this, Brenner astutely fired the question back at Matthaei, asking whether he had any insight into the question. Matthaei said nothing.
29
Above all, the normally garrulous Tomkins had to bite his tongue throughout the Cold Spring Harbor meeting, which took place a week after Brenner’s visit. After the meeting was over, Tomkins finally cracked, and at the end of July he told Alex Rich in Boston. The news went no further, because Rich was too busy with his own work to gossip and, anyway, everyone else was either on holiday or was heading off to the International Biochemical Congress in Moscow.
exactly
what we had. And we knew what we’d wanted to get.’
28
Everyone was sworn to secrecy – nobody was to hear about the finding until the results had been published. This caused some difficulties – at the beginning of June, Sydney Brenner gave a talk at Bethesda, during which he said it was not possible to study messenger RNA in a cell-free system. When Matthaei asked him how he could know this, Brenner astutely fired the question back at Matthaei, asking whether he had any insight into the question. Matthaei said nothing.
29
Above all, the normally garrulous Tomkins had to bite his tongue throughout the Cold Spring Harbor meeting, which took place a week after Brenner’s visit. After the meeting was over, Tomkins finally cracked, and at the end of July he told Alex Rich in Boston. The news went no further, because Rich was too busy with his own work to gossip and, anyway, everyone else was either on holiday or was heading off to the International Biochemical Congress in Moscow.
Matthaei had also found it hard to keep quiet. At the end of June he took the phage course at Cold Spring Harbor, during which each student described his or her research. Matthaei initially refused, but eventually outlined his discovery. Delbrück, who was teaching on the course, was amazed and immediately told Jerry Hurwitz at New York University; Hurwitz in turn phoned Tomkins and got confirmation.
30
The secret was out, and by the beginning of August, researchers in Severo Ochoa’s laboratory in New York had heard the garbled news that ‘someone from MIT’ had broken the code.
31
30
The secret was out, and by the beginning of August, researchers in Severo Ochoa’s laboratory in New York had heard the garbled news that ‘someone from MIT’ had broken the code.
31
Meanwhile, Nirenberg was heading for the Biochemical Congress in Moscow, where he was planning to unveil his discovery. Before he left he had two things to tie up. He got married to Perola Zaltzman, a Brazilian biochemist, and he staked his claim to priority by submitting two articles to
Proceedings of the National Academy of Sciences.
At the time, articles in
PNAS
had to be sponsored by a member of the Academy. Hearing that Academy member Leo Szilárd was staying just down the road in Washington, Nirenberg spent a whole afternoon discussing the results with him in the lobby of the Dupont Hotel. Szilárd was reluctant to help out: ‘It’s too much out of my field,’ he said, ‘I’m sorry, I can’t sponsor it.’
32
It is hard to imagine Szilárd responding in the same way to Jacob or Monod. Nirenberg was clearly an outsider.
Proceedings of the National Academy of Sciences.
At the time, articles in
PNAS
had to be sponsored by a member of the Academy. Hearing that Academy member Leo Szilárd was staying just down the road in Washington, Nirenberg spent a whole afternoon discussing the results with him in the lobby of the Dupont Hotel. Szilárd was reluctant to help out: ‘It’s too much out of my field,’ he said, ‘I’m sorry, I can’t sponsor it.’
32
It is hard to imagine Szilárd responding in the same way to Jacob or Monod. Nirenberg was clearly an outsider.
The two papers were submitted to
PNAS
on 3 August 1961, with the support of Joseph Smadel, the Associate Director of NIH. Straight afterwards, Nirenberg flew to Moscow. The articles appeared, back to back, in the October issue of the journal, by which time everyone who was anyone already knew all about their stunning content. Both papers contained meticulous descriptions of the protocols involved and above all were characterised by the use of tightly conceived control experiments that enabled the authors to exclude alternative explanations, rendering their conclusions incontestable.
PNAS
on 3 August 1961, with the support of Joseph Smadel, the Associate Director of NIH. Straight afterwards, Nirenberg flew to Moscow. The articles appeared, back to back, in the October issue of the journal, by which time everyone who was anyone already knew all about their stunning content. Both papers contained meticulous descriptions of the protocols involved and above all were characterised by the use of tightly conceived control experiments that enabled the authors to exclude alternative explanations, rendering their conclusions incontestable.
The first, more technical, paper described the characteristics of protein synthesis in cell-free
E. coli
extracts, repeating and expanding the results that had been published earlier in the year. Significantly, Matthaei was the first author on this article, which was destined to be read by fewer people (it has been cited fewer than 300 times). This paper showed that protein synthesis could be disrupted by RNase, which attacks RNA, and – eventually and to a lesser extent – by the DNA-destroying enzyme DNase. Matthaei and Nirenberg correctly suggested that the presence of intact RNA was essential for protein synthesis to occur, and that inhibition by DNase was due to ‘the destruction of DNA and its resultant inability to serve as templates for the synthesis of template RNA.’
33
E. coli
extracts, repeating and expanding the results that had been published earlier in the year. Significantly, Matthaei was the first author on this article, which was destined to be read by fewer people (it has been cited fewer than 300 times). This paper showed that protein synthesis could be disrupted by RNase, which attacks RNA, and – eventually and to a lesser extent – by the DNA-destroying enzyme DNase. Matthaei and Nirenberg correctly suggested that the presence of intact RNA was essential for protein synthesis to occur, and that inhibition by DNase was due to ‘the destruction of DNA and its resultant inability to serve as templates for the synthesis of template RNA.’
33
The second paper was obscurely entitled ‘The dependence of cell-free protein synthesis in
E. coli
upon naturally occurring or synthetic polyribonucleotides’. Despite this unappetising opening, it contained the experiment that showed an increase in the amount of radioactive protein when poly(U) was incubated with radioactive phenylalanine – after refining the protocol, they were able to get a roughly 1,000-fold increase over control levels. The article, which has been cited more than 1,400 times, was entirely couched in the language of biochemistry and protein synthesis, referring in a rather old-fashioned way to ‘specificity for phenylalanine incorporation’. Only in the final paragraph did Nirenberg and Matthaei frame their discovery in the new language of life, emphasising that the full detail of the code was not yet known:
E. coli
upon naturally occurring or synthetic polyribonucleotides’. Despite this unappetising opening, it contained the experiment that showed an increase in the amount of radioactive protein when poly(U) was incubated with radioactive phenylalanine – after refining the protocol, they were able to get a roughly 1,000-fold increase over control levels. The article, which has been cited more than 1,400 times, was entirely couched in the language of biochemistry and protein synthesis, referring in a rather old-fashioned way to ‘specificity for phenylalanine incorporation’. Only in the final paragraph did Nirenberg and Matthaei frame their discovery in the new language of life, emphasising that the full detail of the code was not yet known:
One or more uridylic acid residues therefore appear to be the code for phenylalanine. Whether the code is of the singlet, triplet, etc., type has not yet been determined. Polyuridylic acid seemingly functions as a synthetic template or messenger RNA, and this stable, cell-free
E. coli
system may well synthesize any protein corresponding to meaningful information contained in added RNA.
34
Most people assumed that the code was based on triplets, simply because this gave sixty-four possible combinations for twenty naturally occurring amino acids, but this had not been shown to be true. Nirenberg and Matthaei were rightly hedging their bets – strictly speaking, their data were compatible with the unlikely possibility that just a single base of U coded for phenylalanine. Although they again referred to messenger RNA, they did not cite any of the three recently published papers that had first used this term (the two
Nature
papers and the Jacob and Monod review in the
Journal of Molecular Biology,
all of which had appeared in May). Indeed, for reasons that remain obscure, Nirenberg never cited any of these articles.
35
In a note added shortly before the article was printed, they included a recent result obtained by Matthaei while Nirenberg was in Moscow – poly(C) coded for proline. Two words of the genetic code had now been read, but it was still not clear how many letters each contained.*
Nature
papers and the Jacob and Monod review in the
Journal of Molecular Biology,
all of which had appeared in May). Indeed, for reasons that remain obscure, Nirenberg never cited any of these articles.
35
In a note added shortly before the article was printed, they included a recent result obtained by Matthaei while Nirenberg was in Moscow – poly(C) coded for proline. Two words of the genetic code had now been read, but it was still not clear how many letters each contained.*
*
The Fifth International Congress of Biochemistry took place in Moscow, from 10 to 16 August 1961. It was the largest conference ever held in the USSR – there were more than 5,000 participants, including 3,500 foreigners from fifty-eight countries – and was commemorated by a special Soviet postage stamp. With nearly 2,000 talks and up to eighteen parallel sessions, many of the presentations were poorly attended.
36
Eight large symposia, including one organised by Max Perutz on ‘Biological Structure and Function at the Molecular Level’, were held in various Moscow University buildings.
36
Eight large symposia, including one organised by Max Perutz on ‘Biological Structure and Function at the Molecular Level’, were held in various Moscow University buildings.
Other books
Showjumpers by Stacy Gregg
Goodey's Last Stand: A Hard Boiled Mystery (Joe Goodey) by Alverson, Charles
Death Angels by Ake Edwardson
Hunter (Fairy Tale Bad Boys #1) by Erin Bedford
Mystery of the Moss-Covered Mansion by Carolyn Keene
Marked by Death (The Godhunter, Book 4) by Sumida, Amy
Hyde by Tara Brown
Cat and Mouse by William Campbell Gault
Gray's Domain: Purgatorium Series, Book Two by Eva Pohler
Playboy - A Stepbrother Romance by Daire, Caitlin