Power, Sex, Suicide: Mitochondria and the Meaning of Life (16 page)
Read Power, Sex, Suicide: Mitochondria and the Meaning of Life Online
Authors: Nick Lane
Tags: #Science, #General

ATP is often said to have a ‘high-energy’ bond, which is denoted with a ‘squiggle’ (~) rather than a simple hyphen. When broken, this bond is supposed to
release a large amount of energy that can be used to power various forms of work about the cell. Unfortunately, this easy representation is not actually true, for there is nothing particularly unusual about the chemical bonds in ATP. What is unusual is the
equilibrium
between ATP and ADP. There is far,
far
more ATP in the cell, relative to ADP, than there would be if the reaction on
page 79
were left to find its natural equilibrium. If ATP and ADP were mixed together in a test tube and left for a few days, then virtually the entire mixture would break down into ADP and phosphate. What we see in the cell is the absolute reverse: the ADP and phosphate is converted almost totally into ATP. This is a little like pumping water uphill—it costs a lot of energy to pump the water up, but once you have a reservoir on top of a hill, there is a lot of potential energy that can be tapped into later when the water is allowed to rush down again. Some hydroelectric schemes work this way. Water is pumped up to a high reservoir at night when demand is low. It is then released when there is a surge in demand. In England, apparently, there is a massive surge in demand for electricity after popular soap operas, when millions of people go to the kitchen at the same time, and put the kettle on for a nice cup of tea. This surge in demand is met by opening the floodgates of Welsh mountain reservoirs, which are refilled at night after the demand has settled, ready for the next mass teatime.
In the cell, ADP is continually pumped ‘uphill’ to generate a reservoir of potential energy in the form of ATP. This reservoir of ATP awaits the opening of the floodgates, whereupon it is used to power various tasks about the cell, just as the flow of water back downhill is used to power electrical devices. Of course, a lot of energy is needed to produce such a high concentration of ATP, just as a lot of energy is needed to pump water uphill. Providing this energy is the function of respiration and fermentation. The energy released from these processes is used to generate very high cellular levels of ATP in the cell, against the normal chemical equilibrium.
These ideas help us to understand how ATP is used to power work in the cell, but they don’t explain how the ATP is actually formed. The answer seemed to lie in the studies of fermentation by Efraim Racker in the 1940s. Racker was one of the giants of bioenergetics. A Pole by birth, raised in Vienna, he fled the Nazis to Britain at the end of the 1930s, like many of his contemporaries. After internment on the Isle of Man at the outbreak of the war, he moved to the US, and there settled in New York for some years. Deciphering the mechanism of ATP synthesis in fermentation was the first of his many important contributions over fifty years. Racker discovered that, in fermentation, the energy released by breaking down sugars into smaller fragments is used to attach phosphate groups onto the fragments, against a chemical equilibrium. In other words, fermentation generates high-energy phosphate intermediates, and these in turn transfer their phosphates to form ATP. The overall change is energetically
favourable, just as water flowing downhill can be used to turn a waterwheel—the flow of water is
coupled
to the turning of the waterwheel. The formation of ATP likewise takes place via coupled chemical reactions, so that the energy released by fermentation drives a coupled energy-consuming reaction, the formation of ATP. Presumably, thought Racker, and the entire field, a similar model of chemical coupling would also explain how ATP is formed in respiration. Quite the contrary! Rather than offering an insight, it started a wild-goose chase that was to run on for decades. On the other hand, the eventual resolution gave more powerful insights into the nature of life and complexity than anything else in molecular biology, bar the structure of the DNA double helix itself.
The problem hinged on the identity of the high-energy intermediates. In respiration, ATP is produced by a giant enzyme complex called the ATPase (or ATP synthase), which was also discovered by Racker and his colleagues in New York. As many as 30
thousand
ATPase complexes stud the inner mitochondrial membrane, and can be made out faintly down the electron microscope, sprouting like mushrooms from the membrane (
Figure 6
). When first visualized in 1964, Racker described them as the ‘fundamental particles of biology’, an epithet that seems even more apposite today, as we shall see. The ATPase complexes share the inner mitochondrial membrane with the complexes of the respiratory chains, but they are not physically connected to them: they are embedded separately in the membrane. Herein lies the root of the problem. How do these separate complexes communicate with each other across the physical gap? More specifically, how do the respiratory chains transfer the energy released by the flow of electrons to the ATPase, to generate ATP?
In respiration, the
only
known reactions were the redox reactions taking place when electrons were transported down the respiratory chain. The complexes were known to be oxidized and reduced in turn, but that was it: they did not seem to interact with any other molecules. All the reactions were physically separated from the ATPase. Presumably, thought researchers, there must be a high-energy intermediate, as in fermentation, which was formed using the energy released by respiration. This intermediate would then move physically across to the ATPase. After all, chemistry requires contact; action at a distance to a chemist is voodoo. The proposed high-energy intermediate would need to contain a bond equivalent to the sugar-phosphate formed in fermentation, which, when broken, would pass on the energy needed for the high-energy bond of ATP. The ATPase presumably catalysed this reaction.
As so often happens in science, on the threshold of a revolution, the broad outlines seemed to be understood in full. All that remained to be done was to fill in a few details, such as the identity of the high-energy intermediate, which came to be known simply as the squiggle, at least in polite company. Certainly the intermediate was elusive—an entire generation of the finest minds and cleverest experimentalists spent two decades searching for it; they proposed and rejected at least twenty candidates, but even so, finding it only seemed to be a matter of time. Its existence was prescribed by the chemical nature of the cell, little more than a bag of enzymes, as the disciples of Eduard Buchner knew only too well. Enzymes did chemistry, and chemistry was all about the bonds between atoms in molecules.
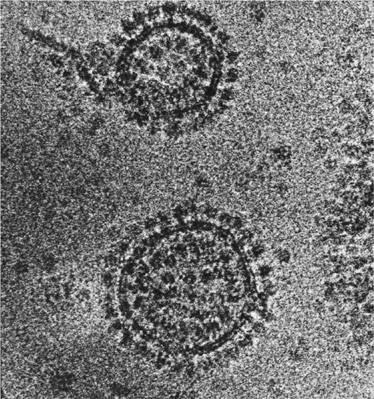
6
The ‘elementary particles of life’ as christened by Efraim Racker. The ATPase proteins sprout like mushrooms on stalks from the membrane vesicles.
But one detail was troubling and nagged at the chemistry of respiration: the number of ATP molecules produced varied. Somewhere between 28 and 38 molecules of ATP are formed from a single glucose molecule. The actual number varies over time, and although it can be as high as 38, it is typically at the lower end of this range. But the important point is the lack of consistency. Because ATP is formed from the passage of electrons down the respiratory chain, the passage of one pair of electrons down the chain generates between 2 and 3 ATPs: not a round number. Chemistry, of course, is all about round
numbers, as anyone knows who has ever struggled to balance chemical equations. It’s not possible to have half a molecule react with two thirds of another molecule. So how could the production of ATP require a variable and non-integer number of electrons?
Another detail also nagged. Respiration
requires
a membrane, and can’t take place at all without it. The membrane is more than just a bag to contain the respiratory complexes. If the membrane is disrupted, respiration is said to become uncoupled, like a bicycle that loses its chain: however furiously we pedal the wheels will not turn. When respiration is uncoupled, the oxidation of glucose via the respiratory chain proceeds apace, but no ATP is formed. In other words, the input is uncoupled from the output and the energy released is dissipated as heat. This curious phenomenon is not simply a matter of mechanical damage to the membrane: it can also be induced by a number of apparently unrelated chemicals, known as
uncouplers
, which do not mechanically disrupt the membrane. All these chemicals (including, interestingly, aspirin and, indeed, ecstasy) uncouple the oxidation of glucose from the production of ATP in a similar fashion, but did not seem to share any kind of chemical common denominator. Uncoupling could not be explained in conventional terms.
By the early 1960s, the field had begun to sink into a slough of despond. As Racker put it (in words reminiscent of Richard Feynman’s celebrated dictum on quantum mechanics): ‘Anyone who is not thoroughly confused just doesn’t understand the problem.’ Respiration generated energy in the form of ATP, but in a manner that did not defer to the basic rules of chemistry, indeed that seemed to flout them. What was going on? Even though these strange findings were crying out for a radical rethink, nobody was prepared for the shocking answer supplied by Peter Mitchell in 1961.
Proton Power
Peter Mitchell was an outsider to the field of bioenergetics. He had studied biochemistry at Cambridge during the war, and began his PhD there in 1943, as he had been injured in a sporting accident before the war and was not enlisted for service. Mitchell was a flamboyant character in the war years, well known about town for his artistic and creative flair, and impish sense of humour. He was an accomplished musician, and liked to wear his hair long in the style of the young Beethoven. Mitchell, too, later fell deaf. His mien was embellished by private means, and he was one of the select few who could afford to drive a Rolls Royce in the drab post-war years; his uncle Godfrey Mitchell owned the Wimpey construction empire. Mitchell’s shares in the company later helped to keep his private research laboratory, the Glynn Institute, afloat. Despite being recognized as one of the brightest young scientists, it took him seven years to finish his PhD, in part because his research was diverted towards wartime goals (the production of antibiotics), and in part because he was asked to resubmit his thesis; one of his examiners had complained that ‘the discussion seemed silly, not a presentation’. David Keilin, who knew Mitchell better, remarked: ‘The trouble is that Peter is too original for his examiners.’
Mitchell’s work concerned bacteria, and especially the problem of how bacteria import and export particular molecules in and out of the cell, very often against a concentration gradient. In more general terms, Mitchell was interested in
vectorial
metabolism, which is to say, reactions that have a direction in space as well as time. The key to bacterial transport systems, for Mitchell, lay in the outer membrane of the bacterial cell. This was plainly not just an inert physical barrier, as all living cells require a continuous and selective exchange of materials across this barrier. At the least, food must be taken up and waste products removed. The membrane acts as a semipermeable barrier, restricting the passage of molecules and controlling their concentration inside the cell. Mitchell was fascinated by the molecular mechanics of active transport across membranes. He appreciated that many membrane proteins are as specific for the molecules that they transport as enzymes are for their raw materials. Also like enzymes, active transport grinds to a halt as the gradient opposing it gathers strength. The force acting to dispel
the gradient strengthens, just as it gets harder to blow air into a balloon as it fills up.
Mitchell developed many of his ideas while at Cambridge in the 1940s and early 1950s, and later in Edinburgh in the late 1950s. He saw active transport as an aspect of physiology, concerning the operation of living bacteria. At that time, there was little intercourse between physiologists and biochemists. Clearly, though, active transport across a membrane requires an input of energy, and this in turn led Mitchell to ponder on bioenergetics, an aspect of biochemistry. He soon realized that if a membrane pump establishes a concentration gradient, then the gradient itself could in principle act as a driving force. Cells could harness such a force in the same way that the escape of air from a balloon can propel it across the room, or the escape of steam can propel a piston in an engine.
These considerations were enough for Mitchell to put forward a radical new hypothesis in
Nature
in 1961, while still at Edinburgh. He proposed that respiration in cells worked by
chemiosmotic coupling
, by which term he meant a chemical reaction that could drive an osmotic gradient, or vice versa.
Osmosis
is a familiar term from schooldays, even if we can’t quite remember what it means. It usually means the flow of water across a membrane from a less concentrated to a more concentrated solution, but Mitchell, characteristically, didn’t mean it in that sense at all. By ‘chemiosmosis’ we might imagine that he was referring to a flow of chemicals other than water across a membrane, but this was not what he meant either. He actually used the word ‘osmotic’ in the original Greek sense, meaning ‘push’. Chemiosmosis, for Mitchell, was the pushing of molecules across a membrane
against
a concentration gradient—it is therefore, in a sense, the exact opposite of osmosis, which follows the concentration gradient. The purpose of the respiratory chain, said Mitchell, is no more nor less than to push protons over the membrane, creating a reservoir of protons on the other side. The membrane is little more than a dam. The pent-up force of protons, trapped behind this dam, can be released a little at a time to drive the formation of ATP.