Power, Sex, Suicide: Mitochondria and the Meaning of Life (12 page)
Read Power, Sex, Suicide: Mitochondria and the Meaning of Life Online
Authors: Nick Lane
Tags: #Science, #General

At present, evidence suggests that the two protagonists of the eukaryotic merger were a methanogen and a metabolically versatile α-proteobacterium, like
Rhodobacter
. The hydrogen hypothesis reconciles the apparently discordant ecological requirements of these protagonists by arguing that the deal revolved around the methanogen’s metabolic addiction to hydrogen, and the bacterium’s
ability to provide it. But for many people this simple solution raises as many questions as it answers. How did a merger that could
only
work under anaerobic conditions (or low oxygen levels) bring about the glorious flowering of the eukaryotes, and especially multicellular eukaryotes, virtually all of which are now totally
dependent
on oxygen? Why did it happen at a time when oxygen levels were rising in the atmosphere and oceans—are we to believe that this was merely coincidence? If the first eukaryote lived in strictly anaerobic conditions, why did it not lose all its oxygen-respiring genes by evolutionary attrition, as anaerobic eukaryotes living today have done? And if the host was not a primitive eukaryotic cell, capable of changing shape and engulfing whole bacteria, how did the α-proteobacteria gain entrance at all?
Along with more recent evidence, the hydrogen hypothesis can explain each of these difficult questions, and remarkably, without even calling for a single evolutionary innovation (the evolution of new traits). Having struggled with these ideas myself, from a position, I should confess, of initial hostility, I believe that something of the sort almost certainly did happen. The chain of events proposed by Martin and Müller has an inexorable evolutionary logic about it, but critically, is dependent on the environment—on evolutionary selection pressures provided by a set of contingent circumstances, which we know happened on Earth at this time. The question is, if the film of life were to be replayed over and over again, as advocated by Stephen Jay Gould, would the same chain of events be repeated? I doubt it, for it seems to me unlikely that the particular train of events proposed by Martin and Müller would repeat itself in a hurry, if at all. My doubts are stronger still for an alien planet, with a different set of contingent circumstances. This is why I suspect that the evolution of the eukaryotic cell was fundamentally a chance event, and happened but once on Earth. Let’s consider what might have happened. I’ll narrate it as a ‘just-so’ story, for clarity, and omit the many ‘may haves’ that clutter the essential meaning (see also
Figure 4
).
Once upon a time, a methanogen and an α-proteobacterium lived side-by-side, deep in the ocean where oxygen was scarce. The α-proteobacterium was a scavenger, which plied its living in many ways, but often generated energy by way of fermenting food (the remains of other bacteria), excreting hydrogen and carbon dioxide as waste. The methanogen lived happily on these waste products, for it could use them to build everything it needed. The arrangement was so cosy and convenient that the two partners grew closer every day, and the methanogen gradually changed its shape (it has a cytoskeleton and shape can be selected for) to embrace its benefactor. Such shape changes can be seen in
Figure 3
.
As time passed, the embrace became quite suffocating, and the poor α-proteobacterium didn’t have much surface left to absorb its food. It would anaerobic respiration die of starvation unless a compromise could be found, but by now it was tightly bound to the methanogen and couldn’t just leave. One possibility would have been for it to physically move inside the methanogen. The methanogen could then use its own surface to absorb all the food needed, and the two could continue their cosy arrangement. So the α-proteobacterium moved in.
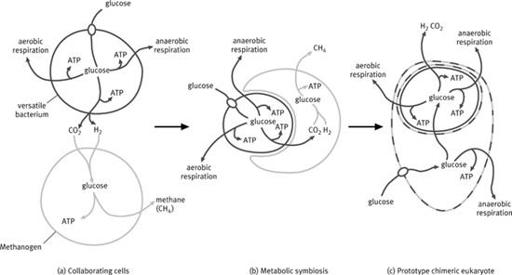
4
Hydrogen hypothesis. Simplified schematic showing the relationship between a versatile bacterium and a methanogen. (a) The bacterium is capable of different forms of aerobic and anaerobic respiration, as well as fermentation to generate hydrogen; under anaerobic conditions the methanogen makes use of the hydrogen and carbon dioxide given off by the bacterium. (b) The symbiosis becomes closer as the methanogen is now dependent on hydrogen produced by the bacterium, which is gradually engulfed. (c) The bacterium is now completely engulfed. Gene transfer from the bacterium to the host enables the host to import and ferment organics in the same way as the bacterium, freeing it from its commitment to methanogenesis. The dashed line indicates that the cell is chimeric.
Before we continue with our just-so story, let’s just note that there are several examples of bacteria living inside other bacteria; it’s not necessary to be phagocytosed. The best-known example is
Bdellovibrio
, a fearsome bacterial predator that moves quickly, at about 100 cell-lengths per second, until it collides with a host bacterium. Just before colliding it spins rapidly and penetrates the cell wall. Once inside, it breaks down the host’s cellular constituents and multiplies, completing its life cycle within 1–3 hours. How many non-predatory bacteria gain access to other bacteria or archaea is a moot question, but the basic postulate of hydrogen hypothesis, that phagocytosis is not necessary to breech another cell, does not sound unreasonable. Indeed, a discovery in 2001 makes it seem more reasonable: mealybugs, the small, white, cotton ball-like insects found living on many house plants, contain α-proteobacteria living within some of their cells as endosymbionts (collaborative bacteria living inside other cells). Incredibly, these endosymbiotic bacteria contain even smaller α-proteobacteria living inside them. Thus one bacterium lives in another, which in turn lives inside an insect cell, showing that bacteria can indeed live peacefully inside one another. The discovery smacks of the old verse: ‘big fleas have little fleas upon their backs to bite ’em; and little fleas have smaller fleas, and so ad infinitum.’
Let’s resume our story. The α-proteobacterium has now found itself inside a methanogen; so far so good. But there was a new problem. The methanogen wasn’t practiced at absorbing its food—it normally made its own from hydrogen and carbon dioxide, and so it couldn’t feed its benefactor after all. Luckily, the α-proteobacterium came to the rescue. It had all the genes necessary for absorbing food, so it could hand them over to the methanogen and all would
be well. The methanogen could now absorb food from outside, and this should have enabled the α-proteobacterium to continue supplying it with hydrogen and carbon dioxide. But the problem was not so easily resolved. The methanogen was now absorbing food, and converting this into glucose on behalf of the α-proteobacterium. The trouble was that methanogens normally use glucose to build up complex organic molecules, whereas the α-proteobacteria break it down for energy. The glucose was subject to a tug of war: instead of passing it on to the greedy bacteria living inside it, which in turn would feed it, the methanogen was inadvertently diverting the food supply to construction projects. If this persisted, both would starve. The α-proteobacterium could solve the problem by handing over more of its genes, which would enable the methanogen to ferment some glucose into breakdown products that the α-proteobacterium could then use. So it handed over the genes.
How, you may be wondering, do unthinking bacteria come to hand over all the genes needed to make such a deal work? This kind of question troubles any discussion of natural selection, but it is answered, as most of them are, simply by thinking about the problem in terms of a population. In this case, we are thinking about a population of cells, some of which thrive, some die, and some continue as they are. Consider a population of methanogens, all of which have many small α-proteobacteria living with them in close proximity. Some individual relationships are relatively ‘distant’, in that the α-proteobacteria are not physically enveloped by the methanogen; they get along fine, but quite a lot of hydrogen is lost to the surroundings, or to other methanogens. These ‘loose’ relationships may lose out to closer relationships, in which the α-proteo-bacteria are being enveloped, and less hydrogen is lost. Of course, each methanogen is likely to harbour a number of α-proteobacteria, a few of which are probably more enveloped than others. So while the overall assemblage might function happily, a few particular α-proteobacteria might be suffocating from the closeness of the embrace. What happens if they die of suffocation? Assuming there are others to take their place, the overall union of the symbiosis might not be affected at all, but the dying α-proteobacterium spills its genes into the environment. Some of these will be taken up by the methanogen in the usual manner by lateral gene transfer, and some will become incorporated into the methanogen’s chromosome. Let’s assume that this process is happening simultaneously in hordes of many millions, perhaps many billions, of symbiotic methanogens. By the law of averages, some at least will happen to transfer all the right genes (which are in any case grouped together as a single functional unit, or an
operon
). If so, then the methanogen will be able to absorb organic compounds from the surroundings. Exactly the same process would account for the transfer of genes for fermentation to the methanogen; and indeed there is no reason why both sets of genes should not be transferred
simultaneously. It’s all down to population dynamics: if the beneficiary happens to be more successful than its brethren, then the power of natural selection will soon amplify the fruit of the successful union.
But there is a startling ending to this story. Having acquired two sets of genes by lateral gene transfer, the methanogen could now do everything. It could absorb food from the surroundings, and ferment it to produce energy. Like an ugly duckling transforming into a swan it suddenly didn’t need to be a methanogen any more. It was free to roam and no longer needed to avoid oxygenated surroundings, which, once upon a time, would have blocked its only source of energy—methane production. What’s more, when roaming in aerobic conditions, the internalized α-proteobacteria could use the oxygen to generate energy much more efficiently, so they too benefited. All that the host (we can’t reasonably call it a methanogen any more) needed was a tap, an ATP pump, which it could plug into the membrane of its α-proteobacterial guest to drain off its ATP, and the entire world would be its stage. The ATP pumps are indeed a eukaryotic invention, and if we are to believe the gene sequences of different groups of eukaryotes, they evolved very early in the history of the eukaryotic union.
So the answer to the question of life, the universe, and everything, or the origin of the eukaryotic cell, was simply gene transfer. Through a series of small and realistic steps, the hydrogen hypothesis explains how a chemical dependency between two cells evolved to become a single chimeric cell containing organelles that function as mitochondria. This cell is able to import organic molecules, like sugars, across its external membrane and to ferment them in its cytoplasm, in the same fashion as yeast. It is able to pass the fermentation products onwards to the mitochondria, which can then oxidize them using oxygen, or for that matter other molecules such as nitrate. This chimeric cell does not yet have a nucleus. It may or may not have lost its cell wall. It does have a cytoskeleton, but has probably not adapted it for changing shape like an amoeba; it merely provides rigid structural support. In short, we have derived a ‘prototype’ eukaryote without a nucleus. We’ll return in
Part 3
to how this prototype might have gone on to become a fully-fledged eukaryote. To end this chapter, though, let’s consider the play of chance in the hydrogen hypothesis.
Each step of the hydrogen hypothesis depends on selection pressures that may or may not have been strong enough to force that particular adaptation, and each step depends utterly on the last—hence the deep uncertainty about whether exactly the same sequence of steps would be repeated if the film of life
were to be played again. For opponents of the theory, the greatest problem lies in the last few steps, the transition from a chemical dependency that only works in the absence of oxygen, to the flowering of the eukaryotic cell as an oxygen-dependent cell which thrives in aerobic conditions. For this to happen, all the genes needed for oxygen respiration must have survived intact throughout the early chimera years, despite falling into prolonged disuse. If the theory is correct then obviously they did; but if the transition had taken just a little longer, then the genes for oxygen respiration may easily have been lost by mutation, and so the oxygen-dependent multicellular eukaryotes would never have been born; and neither would we, nor anything else beyond bacterial slime.
The fact that these genes were not lost sounds like an outrageous fluke, and perhaps this alone accounts for why the eukaryotes only evolved once. But perhaps there was also something about the environment that gave our ancestor a nudge in the right direction. In
Science
, in 2002, Ariel Anbar at the University of Rochester, and Andrew Knoll at Harvard, suggested that the changing chemistry of the oceans might explain why the eukaryotes evolved when they did, at a time of rising oxygen levels, despite their strictly anaerobic lifestyle. As atmospheric oxygen levels rose, so too did the sulphate concentration of the oceans (because the formation of sulphate, SO
4
2–
, requires oxygen). This in turn led to a massive rise in the population of another type of bacteria, the sulphate-reducing bacteria, which we met briefly in
Chapter 1
. There, we noted that the sulphate-reducing bacteria almost invariably out-compete the methanogens for hydrogen in today’s ecosystems, so the two species are rarely found living together in the oceans.