Power, Sex, Suicide: Mitochondria and the Meaning of Life (24 page)
Read Power, Sex, Suicide: Mitochondria and the Meaning of Life Online
Authors: Nick Lane
Tags: #Science, #General

How do
Thermoplasma
survive without a cell wall? Simple: their acidic surroundings fulfil the role of the periplasm, so they have no need of a periplasm of their own. Normally, bacteria pump protons across the external cell membrane into the periplasm outside the cell, which is bounded by the cell wall. This small periplasmic space is therefore acidic, and its acidity is essential for chemi-osmosis. In other words, bacteria normally carry around with them a portable acid bath. In contrast,
Thermoplasma
already live in an acid bath, which is effectively a giant communal periplasm, so they can relinquish their own portable acid bath. As long as they can maintain neutral conditions inside the cell, they can take advantage of the natural chemiosmotic gradient across the cell membrane. So how do they stay neutral inside? Again, the answer is simple: they actively pump protons out of the cell in the same way as any other bacteria, by cell respiration. In other words, as in most prokaryotes, the energy released from food is used to pump protons out of the cell against a concentration gradient; and the backflow of protons into the cell is used to power the ATPase, driving ATP synthesis.
In principle, the absence of a cell wall should not undermine the energetic efficiency or genome size of
Thermoplasma
but in practice the cells are somewhat regressive. Although they can measure up to 5 microns in diameter, their genome, of 1 to 2 million letters, encodes only 1500 genes, and is among the smallest of bacterial genomes; indeed, it is the smallest non-parasitic genome known. Perhaps the extra effort needed to keep out a high concentration of
protons saps the energy that
Thermoplasma
can afford to divert to replicating its genome.
2
Let’s round this up. The exceptions of
Mycoplasma
and
Thermoplasma
only go to prove the rule: the complexity of both bacteria and archaea is curtailed by their need to generate energy across the outer cell membrane. In general, bacteria can’t grow larger because their energetic efficiency falls off quickly as their cell volume increases. If they lose their cell wall, the outer boundary to the periplasm, the proton gradient is more likely to dissipate away, sapping the energy supply and rendering the bacteria fragile. The only prokaryotes that have survived without the cell wall are tiny regressive hermits, such as
Mycoplasma
, which live by parasitism and fermentation, or specialists like
Thermoplasma
, which can only survive in acid. Despite losing their cell walls, and so in principle being able to consume particles, neither group shows any tendency towards the predatory eukaryotic habit of engulfing food by phagocytosis. Neither do they show any tendency to develop a nucleus, or for that matter any other eukaryotic traits. These traits, I shall argue, depend on the possession of mitochondria.
The advantage of mitochondria is that they reside physically inside their host cell. Recall that mitochondria are bounded by two membranes, an outer and an inner membrane, which enclose two distinct spaces, the inner matrix and the inter-membrane space. The respiratory chains and the ATPase complexes are all embedded in the inner mitochondrial membrane, and pump protons from the inner matrix to the inter-membrane space (see
Figure 1
,
page 12
). The acid environment needed for chemiosmosis is therefore contained within the mitochondria and does not affect other aspects of cellular function. (Technically it is not actually acidic, as the protons are buffered, but this doesn’t alter the thrust of the argument.)
Internalization of energy generation within the cell means that an external cell wall is no longer needed, and so can be lost without inducing fragility. Loss of the cell wall frees up the external cell membrane to specialize in other tasks, such as signalling, movement, and phagocytosis. Most importantly of all, internalization releases the eukaryotic cell from the geometric constraints that
oppress bacteria. Eukaryotes are on average 10 000 to 100 000 times the volume of bacteria, but as they become larger, their respiratory efficiency doesn’t slope off in the same way. To increase energetic efficiency, all that eukaryotic cells need to do is to increase the surface area of mitochondrial membranes within the cell; and this can be done simply by having a few more mitochondria. Internalization of energy production therefore enables both the loss of the cell wall and a much greater cell volume. In the fossil record, the sheer size of eukaryotic cells often helps to distinguish them from bacteria—and this greater size appeared quite suddenly, in geological terms, with the internalization of energy generation in the cell. Suddenly, some 2 billion years ago, large eukaryotic cells appear in the fossil record; presumably this must date with some accuracy the origin of the mitochondria, although they themselves can’t be made out in the fossils.
So bacteria are under a strong selection pressure for small size whereas eukaryotes are not. As eukaryotic cells grow larger, they can maintain their energy balance simply by keeping more mitochondria inside—herding more pigs, as it were. So long as they can find enough food to oxidize—enough to feed the pigs—they are not constrained by geometry. Whereas large size is penalized in bacteria, it actually pays dividends in eukaryotes. For example, large size enables a change in behaviour or lifestyle. A large energetic cell does not have to spend all its time replicating its DNA, but can instead spend time and energy developing an arsenal of protein weapons. It can behave like a fungal cell, and squirt lethal enzymes onto neighbouring cells to digest them before absorbing their juices. Or it can turn predator and live by engulfing smaller cells whole, digesting them inside itself. Either way, it doesn’t need to replicate quickly to stay ahead of the competition—it can simply eat the competition. Predation, the archetypal eukaryotic lifestyle, is born of large size, and it depends on overcoming the energetic barriers to being larger. A parallel with human society is the larger communities made possible by farming: with more manpower, it was possible to satisfy food production and still have enough people left over to form an army, or invent lethal new weapons. The hunter-gathers couldn’t sustain such a high population and were bound to lose out to the numerous and specialized competition.
Among cells, it is interesting that predation and parasitism tend to pull in opposite directions. As a rule of thumb, parasites are regressive in character, and in this regard the eukaryotic parasites are no exception. The very word ‘parasite’ conveys something contemptible. Conversely the term ‘predator’ can send shivers up and down the spine. Predation tends to drive evolutionary arms races, in which the predator and prey compete to grow ever larger: the
red queen
effect, whereby both sides must run to stay in the same place, relative to each other. I know of no bacterial cells that are predatory in the eukaryotic fashion
of physically engulfing their prey. Perhaps this should not be surprising. A predatory lifestyle requires a very substantial energetic investment before anything is caught and eaten. At the cellular level, engulfing food by phagocytosis, in particular, demands a dynamic cytoskeleton and an ability to change shape vigorously, both of which consume copious quantities of ATP. So phagocytosis is made possible by three factors: the ability to change shape (which requires losing the cell wall, then developing a far more dynamic cytoskeleton); sufficiently large size to physically engulf prey; and a plentiful supply of energy.
Bacteria can lose their cell wall but have never developed phagocytosis. Vellai and Vida, whom we met earlier, argue that the additional requirements of phagocytosis for large size
and
plentiful ATP may have prevented bacteria from ever becoming effective predators in the eukaryotic style. Respiring over the outer membrane means that bacteria are obliged to generate less energy, relative to their size, as they become bigger. When they become large enough to physically engulf other bacteria they are less likely to have the energy needed to do so. Worse, if the cell membrane is specialized for energy generation, then phagocytosis would also be detrimental, for it would disrupt the proton gradient. It is possible that bacteria could circumvent such problems by relying on fermentation, rather than respiration, as this does not require a membrane. But fermentation also generates substantially less energy than respiration, and this may limit the ability of cells to survive by phagocytosis. Vellai and Vida note that all the eukaryotic cells that live by the combination of fermentation and phagocytosis are parasites, and so might be able to make energy savings in other areas (for example, not synthesizing their own nucleotides and amino acids, the building blocks of DNA and proteins).
3
By sacrificing their energetic expenses in some areas they might be able to justify the energetic costs of phagocytosis. But I’m not aware of any research that looks into this hypothesis systematically, and unfortunately Tibor Vellai has moved on from this field of research.
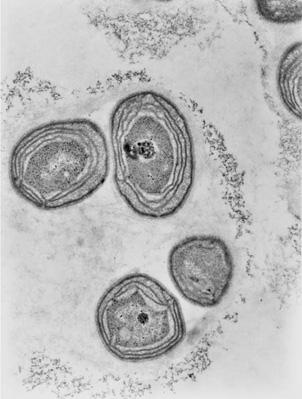
10
Internal bioenergetic membranes of the bacterium
Nitrosomonas
, giving it a ‘eukaryotic’ look.
These ideas are interesting and may go some way towards explaining the dichotomy between bacteria and eukaryotes, but they leave a suspicion at the back of my mind. Why are bacteria
invariably
penalized if they get bigger? Bacteria are so inventive that it is remarkable that none of them have ever solved the challenge of simultaneously increasing their size
and
their energy status. It doesn’t sound so difficult: all they needed to do was grow some internal membranes for generating energy. If internalization of energy production inside the cell enabled eukaryotes to make their quantum leap in size and behaviour, what was to stop bacteria from having internal membranes themselves? Some bacteria, such as
Nitrosomonas
and
Nitrosococcus
do in fact have quite complex internal membrane systems, devoted to generating energy (
Figure 10
). They have a eukaryotic ‘look’ about them. The cell membranes are extensively infolded, creating a large periplasmic compartment. It seems to be a small step from here to a fully compartmentalized eukaryotic cell; so why did it never happen?
In the next chapter, we’ll take up the story of the first chimeric eukaryote that we abandoned without a nucleus at the end of
Part 1
, and look into what may have become of it next. Guided by the principles of energy generation, which we explored in
Part 2
, we’ll see why a symbiosis between two cells was successful, and why, by the same token, it was not possible for bacteria to compartmentalize themselves in the same way as eukaryotes, by natural selection alone. We’ll see why only eukaryotes could become giant predators in a bacterial world—indeed, why they overturned the bacterial world forever.
Why Mitochondria Make Complexity Possible
In the last chapter, we considered why bacteria have remained small and unsophisticated, at least in terms of their morphology. The reasons relate mostly to the selection pressures that face bacteria. These are different from eukaryotic cells because bacteria, for the most part, do not eat each other. Their success in a population therefore depends largely on the speed of their replication. This in turn depends on two critical factors: first, copying the bacterial genome is the slowest step of replication, so the larger the genome, the slower is replication; and second, cell division costs energy, so the least energetically efficient bacteria replicate the slowest. Bacteria with large genomes will always tend to lose out in a race against those with smaller genomes, because bacteria swap genes, by way of lateral gene transfer, and so can keep loading up cassettes of useful genes, and throwing them away again as soon as they become burdensome. Bacteria are therefore faster and more competitive if genetically unburdened.
If two cells have the same number of genes, and have equally efficient energy-generating systems, then the cell that can replicate the fastest will be the smaller of the two. This is because bacteria depend on their outer cell membrane to generate energy, as well as absorbing food. As bacteria become larger in size, their surface area rises more slowly than their internal volume, so their energetic efficiency tails away. Larger bacteria are energetically less efficient, and always likely to lose out in competition with smaller bacteria. Such an energetic penalty against large size precludes phagocytosis, for physically engulfing prey demands both large size and plenty of energy to change shape. Eukaryotic-style predation—catching and physically eating prey—is therefore absent among bacteria. It seems that eukaryotes escape this problem because they generate their energy internally, which makes them relatively independent of their surface area, and enables them to become many thousands of times larger without losing energetic efficiency.
As a distinction between the bacteria and eukaryotes, this reason sounds flimsy. Some bacteria have quite complex internal membrane systems and could be released from the surface-area constraint, yet still don’t approach
eukaryotes in size and complexity. Why not? We’ll look into a possible answer in this chapter, and it is this: mitochondria need genes to control respiration over a large area of internal membranes. All known mitochondria have retained a contingent of their own genes. The genes that mitochondria retain are specific, and the mitochondria were able to retain them because of the nature of their symbiotic relationship with their host cell. Bacteria do not have this advantage. Their tendency to throw away any superfluous genes has prevented them from ever harnessing the correct core contingent of genes to govern energy generation, and this has always prevented them from developing the size and complexity of the eukaryotes.