The Life of Super-Earths (8 page)
Read The Life of Super-Earths Online
Authors: Dimitar Sasselov

Although observations are limited, scientists can study super-Earths today by building theoretical models with a computer, the idea being that they will then be tested by observation. By comparing predictions and observations, we can refine our models even as we learn more about these unusual planets. This is no different from how science studied Earth's interior and the other planets in the Solar System less than 100 years ago. In fact, what we know today about Jupiter-like exoplanets is about equivalent to our knowledge of Jupiter in the 1950s.
Theorists have applied this sort of effort to super-Earths only since 2004. That they were not studied theoretically
before 2004 is a matter of neglect. Theorists usually work on phenomena and objects that are known to exist. Why waste time on something that is not immediately available?
before 2004 is a matter of neglect. Theorists usually work on phenomena and objects that are known to exist. Why waste time on something that is not immediately available?
I am a theorist, so I have to accept some of the responsibility. When Harvard planetary scientist Richard O'Connell and I first talked about computing a model for a planet like Earth but twice as massive, it did not seem to be a difficult project. We were just excited to see what would come out. It was no disappointmentâthe models of these big rocky planets were very interesting indeedâand by the end of the year Diana Valencia, the graduate student Rick had recruited to work on this project, was ready to give a short report at the American Geophysical Union meeting. I was still incredulous that no one had done such models before, so I asked Diana to find out as much as she could by talking to colleagues after her presentation. She came back very encouraged. People were very interested, and no one had computed such models.
7
We were treading new territory, literally!
7
We were treading new territory, literally!
My continuing motivation to find super-Earths had been boosted by experimental success with the method I had worked on for confirming the presence of planets as they transited their stars. The number of confirmed exoplanets continued to grow. And even more exciting was the advent of Kepler.
As a member of the Kepler team and preparing for the mission, I was acutely aware that we were going to discover manyâhundreds ofâplanets smaller than Neptune but bigger than Earth, yet we knew nothing about how their masses and radii should relate to each other. (This was the problem
that had taken me to Rick O'Connell, a colleague but in the Harvard Earth and Planetary Sciences Department, in the first place.)
that had taken me to Rick O'Connell, a colleague but in the Harvard Earth and Planetary Sciences Department, in the first place.)
There are two criteria for calling something a super-Earth, which Diana, Rick, and I established: (1) it is between 1 and 10 M
E
; (2) it is composed mostly of solids (such as rock and ice).
8
This may not seem like a great recipe for variety, and it is true that some of the planets we modeled seemed quite familiar. Far more often, however, we found planets that were exotic and novel.
E
; (2) it is composed mostly of solids (such as rock and ice).
8
This may not seem like a great recipe for variety, and it is true that some of the planets we modeled seemed quite familiar. Far more often, however, we found planets that were exotic and novel.
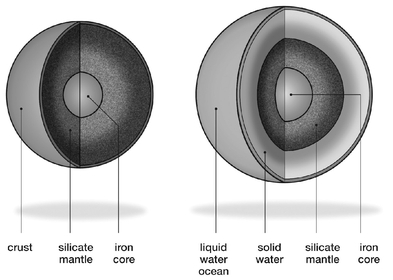
Let's start with the familiar and take a journey to the center of the Earth. The rocky super-Earth cutout in
Figure 5.1
a is a good illustration to the way ahead.
Figure 5.1
a is a good illustration to the way ahead.
The outermost layer is the solid crust. What it's made of depends in part on where we stand. On our own planet, if we begin our journey in California, the crust will be a layer of rocks rich in silica, like granite, that goes about 20 kilometers deep. If we begin our journey on the Pacific Ocean floor near the Hawaiian Islands, the crust will be a layer of basalt rocks denser than silica, such as olivine, that goes only about 5 kilometers deep. On average the Earth's crust is 30 kilometers thick, thinner than that, as we have seen, under oceans, and up to 60 kilometers thick under continental mountain ranges. Our journey to the center is 6,400 kilometers long, so the crust of 30 kilometers is a very brief introduction.
As we go deeper the temperature rises steadily, as miners know all too well, as does the pressure due to all the rocks above us. Consequently, below the crust is a region of partial
melting (the lava of many volcanoes originates there) that quickly becomes the mantle, a thick layer of hot rock, often described as molten. This is actually a misnomer. Yes, on Earth's surface rock at 2,000 degrees flows like liquid from volcanoes, but under the enormous pressure deep in the mantle that same rock is more like cold honey: malleable and extremely slow to flow.
9
The mantle is also in a state that resembles boiling in slow motion (called convection)âthe mantle is so viscous that bubbles in it take millions of years to float to the top.
10
However, this is short compared to the life of the Earth, so on a geological (or planetary) scale of time there is a lot of churning and mixing going on. The temperature at the bottom of Earth's mantle is about 3,700 degrees; that heat is what drives the flow toward the surface. Meanwhile, colder and denser rock near the surface sinks and flows down, dissolving completely in the process. We can see some of the effects of all this churning on the surface, as this convection pushes around the fractured pieces of the crustâknown as the tectonic platesâslowly rearranging the continents.
melting (the lava of many volcanoes originates there) that quickly becomes the mantle, a thick layer of hot rock, often described as molten. This is actually a misnomer. Yes, on Earth's surface rock at 2,000 degrees flows like liquid from volcanoes, but under the enormous pressure deep in the mantle that same rock is more like cold honey: malleable and extremely slow to flow.
9
The mantle is also in a state that resembles boiling in slow motion (called convection)âthe mantle is so viscous that bubbles in it take millions of years to float to the top.
10
However, this is short compared to the life of the Earth, so on a geological (or planetary) scale of time there is a lot of churning and mixing going on. The temperature at the bottom of Earth's mantle is about 3,700 degrees; that heat is what drives the flow toward the surface. Meanwhile, colder and denser rock near the surface sinks and flows down, dissolving completely in the process. We can see some of the effects of all this churning on the surface, as this convection pushes around the fractured pieces of the crustâknown as the tectonic platesâslowly rearranging the continents.
The mantle is Earth's largest layer, some 2,860 kilometers thick, and takes up 84 percent of the planet's volume. The mantle is much denser than the continental rocks, and consists mostly of a mineral called perovskite: a dark dense rock rich in iron and magnesium that is more than 50 percent denser than granite.
11
11
Below the mantle, some 2,900 kilometers below the surface, we encounter the core, consisting of pure iron and iron
alloys. The temperature here is very highâ5,000 to 7,000 Kelvinâcomparable to the temperature on the surface of the Sun. The pressure is very high too. The core is equal in thickness to the mantle, but, being in the center, it is only about 15 percent of the planet's volume. Earth's core consists of two parts: an outer liquid iron core and a solid one below it. Of course, the outer core is liquid in the same sense as the mantle is.
alloys. The temperature here is very highâ5,000 to 7,000 Kelvinâcomparable to the temperature on the surface of the Sun. The pressure is very high too. The core is equal in thickness to the mantle, but, being in the center, it is only about 15 percent of the planet's volume. Earth's core consists of two parts: an outer liquid iron core and a solid one below it. Of course, the outer core is liquid in the same sense as the mantle is.
Â
FIGURE 5.1
.
The interior of a super-Earth. The left image (a) shows the interior of a rocky super-Earth that resembles the interior of the Earth; on the right, the image (b) shows an ocean or water super-Earth with most of its water in a solid form.
.
The interior of a super-Earth. The left image (a) shows the interior of a rocky super-Earth that resembles the interior of the Earth; on the right, the image (b) shows an ocean or water super-Earth with most of its water in a solid form.
We have learned all this from studying earthquakes. The best view of the interior is afforded by analyzing the paths earthquake waves take as they travel through the Earth. Seismographs all over the planet record where and how fast the different waves produced by a single earthquake arrive after bouncing inside the Earth. (This is not too different from the way ultrasound images the inside of the human body from outside.) The full picture is put together by adding to this our knowledge of Earth's magnetic field, heat flow, gravity studies from spacecraft, and laboratory experiments on rocks under high pressure.
I alluded to how some of these structures form in Chapter 2, but the full picture is a bit more complicated than I've explained thus far. The preplanet structureâthe “seed” of a planetâconsists of solids (mostly silicates) and volatiles (such as water and ammonia), with trace amounts of hydrogen and noble gases. Due to the energy of the accretion process and the constant collisions with large solid bodies, this seed is thoroughly molten. (Some of Earth's internal heat is a relic of this process.) In this state the structure differentiates.
Iron and siderophile elements (high-density transition metals that like to bond with iron) precipitate from the silicate mix and sink under their own weight to form the core in the center. The remaining silicate minerals will remain in a mantle with the less dense ones closer to the top. Volatiles that are left over after hydrating the mantle minerals will rise to the surface and atmosphere.
Iron and siderophile elements (high-density transition metals that like to bond with iron) precipitate from the silicate mix and sink under their own weight to form the core in the center. The remaining silicate minerals will remain in a mantle with the less dense ones closer to the top. Volatiles that are left over after hydrating the mantle minerals will rise to the surface and atmosphere.
This process, called planetary differentiation, is quick in geological terms and works for planetary bodies as small as big asteroids just a few miles across. Iron meteoritesâpieces of pure iron alloy that orbit around the Sun until one day they fall to Earth for us to find themâoriginate in the differentiated iron cores of asteroids that were later smashed up by collisions with other asteroids. So, although we have no samples of our Earth's iron core, and no good prospects to get them anytime soon, iron meteorites are excellent proxies. Differentiation is an orderly and predictable process thanks to our knowledge of chemistry and mineral properties under pressure.
Some super-Earths, the rocky ones, develop quite similarly, although the pressure in the mantle is almost tenfold higher and different varieties of minerals form. Other super-Earths, the oceanic ones, are totally exotic beasts, with oceans that are 100 kilometers deep overlying a dense hot solid water, called ice VII.
It might seem ridiculous to refer to this water as ice, given that it is at a searing temperature of 1,000 K, but under such high pressures, it forms. WaterâH
2
Oâhas a familiar structure and formula, but our familiarity with it can make us overlook the fact that it is actually very complex. One key feature
is that the oxygen atom in its molecule does not share electrons equally with its two hydrogen atoms; the result is that the molecule ends up with an asymmetric distribution of electrical charge. Imagine the tiny water molecules like small magnets, except with three poles (a negative O and two positive H's). Water molecules interact with each other because the positive charge near a hydrogen atom of one molecule bonds weakly with the negative charge near the oxygen atom of another molecule. Many such weak bonds together can form a strong structure if the temperature becomes low enough to allow it. Thus common water ice is formed, dubbed ice Ih or hexagonal ice. In common ice the weak bonds between the molecules cause the molecules to form rings (mostly hexagons) that leave lots of empty space in between. The empty space gives it a lower density than liquid water, and soâas you know from a glass of ice waterâit floats.
2
Oâhas a familiar structure and formula, but our familiarity with it can make us overlook the fact that it is actually very complex. One key feature
is that the oxygen atom in its molecule does not share electrons equally with its two hydrogen atoms; the result is that the molecule ends up with an asymmetric distribution of electrical charge. Imagine the tiny water molecules like small magnets, except with three poles (a negative O and two positive H's). Water molecules interact with each other because the positive charge near a hydrogen atom of one molecule bonds weakly with the negative charge near the oxygen atom of another molecule. Many such weak bonds together can form a strong structure if the temperature becomes low enough to allow it. Thus common water ice is formed, dubbed ice Ih or hexagonal ice. In common ice the weak bonds between the molecules cause the molecules to form rings (mostly hexagons) that leave lots of empty space in between. The empty space gives it a lower density than liquid water, and soâas you know from a glass of ice waterâit floats.
Under high pressure the density of water increases as the molecules are forced closer together; the bonds are bent to form tighter rings, which also interpenetrate. That makes the water solid, almost irrespective of the temperature, and much denser than the liquid phase.
12
The high-pressure ices that exist at high temperatures are known as ice VII, X, and XI; these are the ice phases we expect to find inside oceanic super- Earths.
13
These ices are still less dense than rocks, however, so an oceanic super-Earth will be less dense than a rocky one of the same mass.
12
The high-pressure ices that exist at high temperatures are known as ice VII, X, and XI; these are the ice phases we expect to find inside oceanic super- Earths.
13
These ices are still less dense than rocks, however, so an oceanic super-Earth will be less dense than a rocky one of the same mass.
Ocean planets might be very common in the Universe because water is very common in the low-temperature environments where planets form and evolve.
14
This might be
especially true for super-Earths, which can retain volatiles more easily thanks to their larger mass and surface gravity.
15
In order for a planet to become an ocean planet, it should form with or obtain at least 10 percent of its mass in water. Ammonia could be mixed in, but water is by far the dominant volatile chemical we see among the materials in protoplanetary disks. For comparison, Earth's oceans are just about 0.02 percent of its mass. However, a much greater amount of water could be incorporated into Earth's mantle.
16
For that reason I assume a much larger fraction of water (greater than 10 percent) to produce a separate uninterrupted layer of water surrounding a planet (
Figure 5.1
b).
14
This might be
especially true for super-Earths, which can retain volatiles more easily thanks to their larger mass and surface gravity.
15
In order for a planet to become an ocean planet, it should form with or obtain at least 10 percent of its mass in water. Ammonia could be mixed in, but water is by far the dominant volatile chemical we see among the materials in protoplanetary disks. For comparison, Earth's oceans are just about 0.02 percent of its mass. However, a much greater amount of water could be incorporated into Earth's mantle.
16
For that reason I assume a much larger fraction of water (greater than 10 percent) to produce a separate uninterrupted layer of water surrounding a planet (
Figure 5.1
b).
An ocean planet, regardless of its surface temperature, should have the same layers inside: an iron core surrounded by a silicate-rich mantle that transitions into the hot water ice. The latter will become liquid water near the surface (the last 100 kilometers or so). The surface of the liquid water ocean will be covered with ice Ih, if the planet is far from its star and cold, like Jupiter's moon Europa. If the planet is close to its star and hot at the surface, the liquid ocean will transition into a thick hot steam atmosphere. If the planet has moderate temperatures such as we have on Earth, the water ocean will resemble Earth's, but there will be no continents or basalt tectonic plates under it. The interior of the ocean planet will remain under the control of the planet's internal reservoir of heat. The transition between silicate mantle and hot water ice happens with a small change in density but no change in temperature, and the two materials
have similarly high viscosity. Like the silicate mantle, the hot water ice “mantle” convects slowly.
have similarly high viscosity. Like the silicate mantle, the hot water ice “mantle” convects slowly.
The two families of super-Earths have planets that are diverse in size and amount of water. These characteristics depend on the mixture of elements present as the planet forms. From studying the spectra of many stars, we know that the amount of iron and other heavy elements will be different in different planetary systems. We already know that where in the proto-planetary disk a planet forms also matters. So, among the rocky planets we could find super-Mercurysâplanets that have as much as 70 percent of iron core inside, like our planet Mercury.
17
Or we could find super-Moonsâplanets that have no iron core, just an iron-rich mantle and perhaps a water layer.
18
17
Or we could find super-Moonsâplanets that have no iron core, just an iron-rich mantle and perhaps a water layer.
18
Other books
World of Warcraft: Chronicle Volume 1 by BLIZZARD ENTERTAINMENT
Legends of Marithia: Book 3 - Talonsphere by Peter Koevari
Double Down: Game Change 2012 by Mark Halperin, John Heilemann
Indelible by Woodland, Lani
Reconstructing Amelia by Kimberly McCreight
A Perfect Night by Unknown
Cogs in Time Anthology (The Steamworks Series) by Stovall, Catherine, Clark, Cecilia, Gatton, Amanda, Craven, Robert, Ketteman, Samantha, Michaels, Emma, Marlow, Faith, Stevens, Nina, Staum, Andrea, Adams, Zoe, S.J. Davis, D. Dalton
The Devil and Sonny Liston by Nick Tosches
Unexpected Magic by Diana Wynne Jones
Her Demonic Angel (Her Angel Romance Series Book 5) by Felicity Heaton