Your Inner Fish: A Journey Into the 3.5-Billion-Year History of the Human Body (26 page)
Read Your Inner Fish: A Journey Into the 3.5-Billion-Year History of the Human Body Online
Authors: Neil Shubin

We can thank our shared history with sharks and fish for this. If you have ever tried to catch a trout, then you have come up against an organ that is likely an antecedent to our inner ear. As every fisherman knows, trout hold only in certain parts of a stream, typically spots where they can get the best meal while avoiding predators. Often such places are in the shade and in the eddies of the stream’s current. Great places for big fish to hold are behind big rocks or fallen logs. Trout, like all fish, have a mechanism that allows them to sense the current and the motion of the water around them, almost like a sense of touch.
Within the skin and bones of the fish, arranged in lines that run the length of the body and head, are small organs with sensory receptors. These receptors lie in small bundles from which they send small hair-like projections into a jelly-filled sac called a neuromast organ. It helps to think of the snow globe Statue of Liberty again. A neuromast organ is like a tiny one of these, with nerves projecting inside. When the water flows around the fish, it deforms this small sac, thereby bending the hair-like projections of the nerve. Much like the whole system in our ears, this apparatus then sends a signal back to the brain and gives the fish a sense of what the water is doing around them. Sharks and fish can discern the direction in which the water is flowing, and some sharks can even detect distortions of the water, such as are produced by other fish swimming near them. We used a version of this system when we moved our head with a fixed gaze, and we saw it go awry when we propped open the eyes of the inebriated individual at the start of this section. If the ancestor we have in common with sharks and fish had used some other kind of inner ear gel, say one that does not swirl when alcohol is added, we would never spin when drunk.
If you think of our inner ears and neuromast organs as versions of the same thing, you would not be far off. Both come from the same sort of tissue during development, and they share a similar structure. But which came first: neuromasts or inner ears? Here the evidence gets sketchy. If you look at some of the earliest fossils with heads, creatures about 500 million years old, you’ll find little pits in their external armor that suggest they had neuromast organs. Unfortunately, we do not know much about the inner ears of these creatures because the preservation of that area of the head is wanting. Until more evidence rolls in, we are left with one of two alternatives: either our inner ears arose from neuromast organs or the other way around. Both scenarios, at their core, reflect a principle we’ve seen at work in other parts of the body. Organs can come about for one function, only to be repurposed over time for any number of new uses.
In our own ears, there occurred an expansion of the inner ear. The part of our inner ear devoted to hearing is, as in other mammals, huge and coiled. More primitive creatures, such as amphibians and reptiles, have a simple uncoiled inner ear. Clearly, our mammalian forebears obtained a new and better type of hearing. The same is true for the structures that perceive acceleration. We have three canals to record acceleration because we perceive space in three dimensions. The earliest known fish with these canals, a kind of jawless fish like a hagfish, has only one. Then, in other primitive fish, we see two. Finally, most modern fish, and other vertebrates, have three, like us.
We have seen that our inner ear has a history that can be traced to the earliest fish. Remarkably, the neurons inside the gel of our ears have an even more ancient history.
These neurons, called hair cells, have special features that are seen in no other neuron. With fine hair-like projections, consisting of one long “hair” and a series of smaller ones, these neurons lie with a fixed orientation in our inner ear and in a fish’s neuromast organ. Recently, people have searched for these cells in other creatures, and have found them not only in animals that do not have sense organs like ours at all but also in animals that have no heads. They are seen in creatures like
Amphioxus,
which we met in Chapter 5, that have no ears, eyes, heads, or skulls. Hair cells, then, were around doing other things before our sense organs even hit the scene.
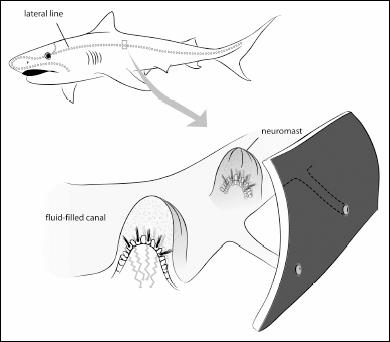
A primitive version of part of our inner ear is embedded in the skin of fish. Small sacs—the neuromasts—are distributed around the body. When they bend, they give the fish information about how the flow of water is changing.
All this is recorded in our genes, of course. If humans or mice have a mutation that knocks out a gene called
Pax 2,
the inner ear fails to form properly.
Pax 2
is active in the ear region and appears to start a chain reaction of gene activity that leads to the development of the inner ear. Go fishing for this gene in more primitive animals and we find
Pax 2
active in the head and, lo and behold, in the neuromasts. The spinning drunk and the fish’s water-sensing organs have common genes: evidence of a common history.
JELLYFISH AND THE ORIGINS OF EYES AND EARS

Just like
Pax 6,
which we discussed earlier in connection with eyes,
Pax 2
in ears is a major gene, essential for proper development. Interestingly, a link between
Pax 2
and
Pax 6
suggests that ears and eyes might have had a very ancient common history.
This is where the box jellyfish enters our story. Well known to swimmers in Australia because they have particularly poisonous venom, these jellyfish are different from most others in that they have eyes, more than twenty of them. Most of these eyes are simple pits spread over the jellyfish’s epidermis. Other eyes on the body are strikingly similar to our own, with a kind of cornea, a lens, even a nervous structure like ours.
Jellyfish do not have either
Pax 6
or
Pax 2:
they arose before those genes hit the scene. But in the box jellyfish’s genes we see something remarkable. The gene that forms the eyes is not
Pax 6,
as we’d expect, but a sort of mosaic that has the structure of
both Pax 6
and
Pax 2.
In other words, this gene looks like a primitive version of other animals’
Pax 6
and
Pax 2.
The major genes that control our eye and ear correspond to a single gene in more primitive creatures, such as jellyfish. You’re probably thinking, So what? The ancient connection between ear and eye genes helps to make sense of things we see in hospital clinics today: a number of human birth defects affect
both
the eyes and the inner ear. All this is a reflection of our deep connections to primitive creatures like the stinging box jellyfish.
CHAPTER ELEVEN

THE MEANING OF IT ALL
THE ZOO IN YOU

My professional introduction to academia happened in the early 1980s, during my college years, when I volunteered at the American Museum of Natural History in New York City. Aside from the excitement of working behind the scenes in the collections of the museum, one of the most memorable experiences was attending their raucous weekly seminars. Each week a speaker would come to present some esoteric study on natural history. Following the presentation, often a fairly low-key affair, the listeners would pick the talk apart point by point. It was merciless. On occasion, the whole thing felt like a human barbecue, with the invited speaker as the spit-roasted main course. Frequently, these debates would devolve into shouting sessions with all the high dudgeon and operatic pantomime of an old silent movie, complete with shaken fists and stomped feet.
Here I was, in the hallowed halls of academe, listening to seminars on taxonomy. You know, taxonomy—the science of naming species and organizing them into the classification scheme that we all memorized in introductory biology. I could not imagine a topic less relevant to everyday life, let alone one less likely to lead eminent senior scientists into apoplexy and the loss of much of their human dignity. The injunction “Get a life” could not have seemed more apt.
The irony is that I now see why they got so worked up. I didn’t appreciate it at the time, but they were debating one of the most important concepts in all of biology. It may not seem earth-shattering, but this concept lies at the root of how we compare different creatures—a human with a fish, or a fish with a worm, or anything with anything else. It has led us to develop techniques that allow us to trace our family lineages, identify criminals by means of DNA evidence, understand how the AIDS virus became dangerous, and even track the spread of flu viruses throughout the world. The concept I’m about to discuss supplies the underpinning for much of the logic of this book. Once we grasp it, we see the meaning of the fish, worms, and bacteria that lie inside of us.
The articulation of truly great ideas, of the laws of nature, begins with simple premises that all of us see every day. From simple beginnings, ideas like these extend to explain the really big stuff, like the movement of the stars or the workings of time. In that spirit, I can share with you one true law that all of us can agree upon. This law is so profound that most of us take it completely for granted. Yet it is the starting point for almost everything we do in paleontology, developmental biology, and genetics.
This biological “law of everything” is that every living thing on the planet had parents.
Every person you’ve ever known has biological parents, as does every bird, salamander, or shark you have ever seen. Technology may change this, thanks to cloning or some yet-to-be-invented method, but so far the law holds. To put it in a more precise form: every living thing sprang from some parental genetic information. This formulation defines parenthood in a way that gets to the actual biological mechanism of heredity and allows us to apply it to creatures like bacteria that do not reproduce the way we do.