Read Life's Ratchet: How Molecular Machines Extract Order from Chaos Online
Authors: Peter M. Hoffmann
Life's Ratchet: How Molecular Machines Extract Order from Chaos (32 page)

FIGURE 7.5.
The waiting state of a kinesin motor protein on top of a microtubule. The cargo domain has been omitted for simplicity.
Now, an ATP molecule floats by. It can’t bind to the trailing, dangling head (its binding pocket is already occupied by ADP), but the empty head in front is happy to take in the lonely ATP. Binding ATP releases energy, and in this case, the energy is used to dock the neck linker with the leading head. This tilts the molecule forward and produces some strain between the two heads (which, after all, are linked by a rather short neck). By a combination of the strain in the linker and, possibly, the tilted energy landscape provided by the microtubule, the dangling head performs what is called
biased diffusion
. That is, the head is pushed about by the molecular storm, and since the energy landscape is tilted in the forward direction, the head moves forward—most of the time (but not always—sometimes it will step backward!).
This diffusive search for a low-energy landing place is still a bit of a mystery. Some research groups have determined that the docking of the neck linker is associated with an energy that is comparable with the energy contained in the molecular storm. This would not be enough to resist the onslaught of marauding water molecules. However, other researchers found that ATP binding may provide a much higher amount of energy,
around thirteen times the thermal energy of the molecular storm. Comfortable sites on which the head could land are separated by 8 nm. The site directly ahead is already occupied by the leading head, so the dangling head has to diffuse 16 nm to find a new, comfortable resting place, an appreciable distance for a molecular motor. The dangling head must step around the planted head, walking like a cowboy wearing chaps, to find its resting place. It is difficult to see how a small forward tilt of maybe 1–2 nm due to the docking could propel the head a full 16 nm. Yanagida’s group, of myosin II controversy fame, measured how kinesin steps at different temperatures and found an entropy contribution worth four times the energy of the molecular storm. Thus, the missing component may be entropy; in other words, the forward motion is entropically favored over backward motion.
Once the searching head finds it new resting place, 16 nm from where it started, it releases its ADP and tightly binds to the microtubule. The empty binding pocket of this head is now ready to bind an ATP. The molecule has taken one step, the leading head is now trailing, and the formerly dangling head is now leading. But we are not yet back to square one: The now trailing head is still bound to ATP. Through an allosteric interaction, the ATP in the trailing head keeps the new leading head from binding an ATP. This is a good thing. If the leading head were to bind ATP while the rear head was still attached to the track, it would dock the neck linker and introduce intolerable strain in the molecule. Instead, the trailing head first hydrolyzes its ATP, splitting off a phosphate ion and turning ATP into ADP. Once this happens, the trailing head detaches, the leading head is free to bind ATP, and the cycle repeats (
Figure 7.6
). We can see from this sequence how stepping is choreographed: The motion controls the chemical transitions, and the chemical transitions control the motion through allostery. This chemomechanical coupling ensures that the motor never falls out of step. It is this tight control of the motion that makes kinesin a highly processive motor.
Let us relate this detailed scheme to the more general ideas introduced in the previous chapters. There we saw that we need a tilted energy landscape and a supply of free energy to reset the system. In kinesin, the tilted energy landscape comes from the strain induced between the two heads by the short neck linker, the binding energy of the microtubule, and, possibly,
an entropy contribution. Free energy is supplied when ATP binds, when the head binds to the microtubule, and when ATP is hydrolyzed. Some of this energy is used to dock the neck linker and produce strain, which in turn imparts kinetic energy to the dangling head. Some energy must also be used to detach from the microtubule and to release spent ADP. Eventually all energy will lead to a vibration of the kinesin and microtubule and end up as waste heat. The result is that free energy, in the form of ATP, is used to do some work, and the rest of the energy is dissipated as heat. No violation of the second law here.
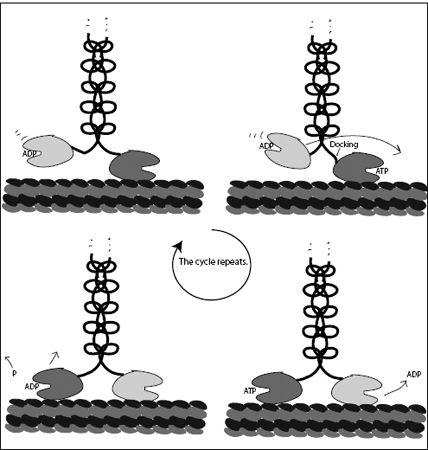
FIGURE 7.6.
Left to right: The step-by-step motion of kinesin-1. At the end of the sequence, the cycle repeats, with the two heads having traded places and the molecular motor having advanced one step.
What is striking, however, is that the main source of energy, the hydrolysis of ATP, does not seem to be directly used for locomotion. Rather, as in the case of Maxwell’s demon, or the nano-Sisyphus, it provides a reset step. The energy in ATP is used to detach the trailing head from the microtubule and thus, in some sense, erase the information about where the trailing head was previously attached. Kinesin is a tightly coupled motor— it takes one ATP to take one step. It holds on tight when it must, but when it steps, it acts like a Brownian ratchet, diffusing on an energy landscape that is sculpted not only by the environment, but also by mechanical strains generated inside the molecule itself. What an ingenious little machine!
THE FAMILY OF KINESINS
We have so far only talked about one type of kinesin: kinesin-1. As mentioned, there are ten classes of kinesins, and each class has many members. Humans have forty-five different kinesins encoded in our DNA. There are many types of kinesin because they all have different jobs and work a little bit differently. But they are all kinesins, because they share an identical motor domain and they are all molecular motors.
The location of the motor domain in relation to the rest of the protein varies greatly between the different kinesins. In general, proteins have two ends. Proteins are folded strings of amino acids, and molecular motors are no different. Each amino acid has a carboxyl group at one end and an amino group on the other. When the amino acids link together, the carboxyl end of one amino acid hooks up with the amino end of the other, forming what is called a peptide bond. But that leaves a carboxyl group at one end and an unbounded amino group at the other end. As more and more amino acids are added, there is always a carboxyl end (called the C terminus) and an amino end (called the N terminus—because amino groups contain nitrogen). Kinesins can be classed into three groups, depending on where the motor protein is located: near the C terminus, near the N terminus, or somewhere in between. Most kinesin molecules are N-terminal, but there are three C-terminal and three M (middle) kinesins in humans. Interestingly, N-terminal kinesins walk along microtubules toward what is called the plus end (toward the cell wall, away from the interior of the cell), while C-terminal kinesins walk toward the minus end (toward the cell center).
Kinesins transport vesicles (lipid-enclosed sacks), which can contain various molecules, or they create tension in microtubule networks, especially during cell division. Some kinesins are active only in the cells of our nervous system, and indeed, mutations to the amino acid sequence of these kinesins are linked to neurological diseases. Some kinesins move whole organelles (substantial subunits of the cell). For example, a kinesin called KIF1B moves mitochondria in nerve cells. As mentioned earlier, mitochondria are the cell’s power plants, which recharge ADP to ATP. Other kinesins (e.g., kinesin-2), have nonidentical motor domains and play important roles in embryonic development. Chromokinesin is involved in moving DNA around during cell division. When cells divide, the DNA information has to be equitably distributed between the two daughter cells. This is achieved by separating the chromosomes (which are bundled up DNA) in a complicated process called mitosis. This separation is accomplished by armies of molecular machines, kinesins among them.
Two of the more bizarre kinesins are kinesin-3 and kinesin-5. Kinesin-3 appears to only have one head and a loop to hold on to a microtubule. Kinesin-3 has been suggested as an example of a Brownian ratchet–based motor, because a single head cannot really move the way kinesin-1 moves; how could strain be generated if no part of the molecule is attached to the microtubule? In this model, the loop may help to hang on to the microtubule while the head detaches for (directed) diffusion. However, recent research has suggested that kinesin-3 molecules pair up and work together, much like the two permanently connected heads in a kinesin-1 molecule.
Kinesin-5 represents the opposite extreme: It has four heads, two pairs back-to-back. Kinesin-5 is involved in mitosis. It attaches to two microtubules at the same time and pulls them together. During mitosis, the chromosomes are attached to a structure called the spindle, which is made of microtubules. Kinesin-5 is the motor that keeps the spindle taut.
MYOSIN V: RELATING MOTION AND CHEMISTRY
In 2009, about a year into a new undergraduate program—biomedical physics—at Wayne State University, our department hired a biophysicist. Through a stroke of luck, it turned out to be my colleague Takeshi
Sakamoto. Takeshi specializes in molecular motors, with several ground-breaking works to his credit. And his baby is myosin V.
Myosin V, like kinesin, is a walking transport motor. There are some differences, however: Myosin moves on actin filaments rather than microtubules, and it has long legs, rather than a short neck. Thus, it strides, rather than waddles. While kinesin and dynein are the long-distance trains of the cell, myosin V is more like a local transporter. It takes cargo from kinesin and moves it a short distance to the cell membrane, where the cargo is passed off to other molecular machines, which then move the cargo out of the cell.
At the 2011 Biophysical Society Meeting, I met Takeshi’s Ph.D. advisor, who is from Kanazawa University. Toshio Ando blamed Takeshi for getting him hooked on myosin V. Ando was not too unhappy about this addiction. His fascination with molecular motors drove him to create one of the most sophisticated atomic force microscopes in the world. Ando’s AFM can scan so fast that a single image is completed in twenty milli-seconds. This may not mean much to the uninitiated, but AFMs usually take tens of seconds or even minutes to complete one image. As described earlier, AFMs create an image by moving a sharp tip over a surface, and if you move the tip too fast, you will tear your sample to shreds. To move it as fast as Ando does, you need superfast electronics and hardware. It took Ando and his students almost ten years to perfect their technique.
Ando showed his now-iconic movie of a walking myosin V molecule at the biophysical meeting. Using his high-speed AFM, Ando and his students filmed single myosin molecules as they walked along actin filaments. These are mesmerizing little movies: They are fuzzy and the images wobble, but you can easily see a nanometer-size machine, like a little two-legged creature, wait, then suddenly do a quick step, then wait again, step again, and so on. Is this molecule alive? No, not in the full sense of the word. But watching it stride by, you can see how many such machines, interacting in some regulated way, can make a living being. This surely is where life begins.
Takeshi left Ando’s group before they made this movie, but he joined another well-known group studying myosin V: Jim Sellers’s group at the National Institutes of Health in Bethesda, Maryland. Sellers had been studying myosins (especially myosin II, muscle myosin) since he completed his
Ph.D. in 1980. Takeshi worked in Sellers’s group for eight years before joining Wayne State. His crowning achievement was the simultaneous imaging of the motion of a walking myosin V molecule and its uptake of ATP. This study was the holy grail: the linking of the biochemical cycle (ATP binding, hydrolysis, and ADP release) with the mechanical cycle (detaching, diffusing, stepping, reattaching). Linking the two in space and time on a single molecule goes a long way toward understanding how these molecules convert chemical energy into mechanical energy. This had never been done, not for kinesin or any of the other molecular motors.
To link the chemical and mechanical cycles, Takeshi used an ATP with an attached fluorescent label. This label shone up to twenty-five times brighter when the labeled ATP bound to myosin. Thus, Takeshi and coworkers could easily tell if the nucleotide (ATP or ADP) was bound to a myosin head. To follow the motion of the myosin, they used another label attached directly to the body of the myosin. Takeshi found that myosin V was a tightly coupled motor—one ATP, one step. Kinesin is also believed to be a tightly coupled motor, but this is still not completely proven. Thus, Takeshi’s experiments were the first to clearly demonstrate, for any molecular machine, a one-to-one relationship between ATP hydrolysis and motion. This was an important finding, because after Yanagida’s work, it seemed possible that myosin V’s cousin, myosin II, was weakly coupled, moving variable distances for each ATP consumed.
