Power, Sex, Suicide: Mitochondria and the Meaning of Life (4 page)
Read Power, Sex, Suicide: Mitochondria and the Meaning of Life Online
Authors: Nick Lane
Tags: #Science, #General

The DNA code is inert, a vast repository of information housed out of the way in the nucleus, in the same way that valuable encyclopaedias are stored safely in libraries, rather than being consulted in factories. For daily use the cell relies on disposable photocopies. These are made of RNA, a molecule composed of similar building blocks to DNA, but spun-out in a single strand rather than the two strands of the double helix. There are several types of RNA, which fulfil distinct tasks. The first of these is messenger RNA, which equates in length, more or less, to a single gene. Like DNA, it, too, forms a string of letters, and their sequence is an exact replica of the gene sequence in the DNA. The gene sequence is
transcribed
into the slightly different calligraphy of messenger RNA, converted from one font into another without losing any meaning. This RNA is a winged messenger, and passes physically from the DNA in the nucleus, through the pores that pockmark its surface like the moon, and out into the cytoplasm. There it docks onto one of the many thousands of protein-building factories in the cytoplasm, the
ribosomes
. As molecular structures these are enormous; as visible entities they are miniscule. They can be seen studding some of the cell’s internal membranes, giving them a rough impression on the electron microscope, and dotting through the cytoplasm. They are composed of a mixture of other types of RNA, and protein, and their job is to
translate
the message encoded in messenger RNA into the different language of proteins—the sequence of amino acids. The whole process of transcription and translation is controlled and regulated by numerous specialized proteins, the most important of which are called
transcription factors
. These regulate the expression of genes. When a gene is expressed, it is converted from the somnolent code into an active protein, with business about the cell or elsewhere.
Armed with this basic cell biology, let’s now return to the mitochondria. They are organelles in the cell—one of the tiny organs dedicated to a specific task, in this case energy production. I mentioned that mitochondria were once bacteria, and in appearance they still look a bit like bacteria (
Figure 1
). Typically depicted as sausages or worms, they’re able to take many twisted and contorted shapes, including corkscrews. They’re usually of bacterial size, a few thousandths of a millimetre in length (1 to 4 microns), and perhaps half a micron in diameter. The cells that make up our bodies typically contain numerous mitochondria, the exact number depending on the metabolic demand of that particular cell. Metabolically active cells, such as those of the liver, kidneys, muscles, and brain, have hundreds or thousands of mitochondria, making up some 40 per cent of the cytoplasm. The egg cell, or oocyte, is exceptional: it passes on around 100000 mitochondria to the next generation. In contrast, blood cells and skin cells have very few, or none at all; sperm usually have fewer than 100. All in all, there are said to be 10 million billion mitochondria in an adult human, which together constitute about 10 per cent of our body weight.
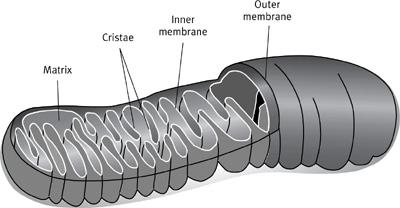
1
Schematic representation of a single mitochondrion, showing the outer and inner membranes; the inner membrane is convoluted into numerous folds known as cristae, which are the seat of respiration in the cell.
Mitochondria are separated from the rest of the cell by two membranes, the outer being smooth and continuous, and the inner convoluted into extravagant folds or tubules, called
cristae
. Mitochondria don’t lie still, but frequently move around the cell to the places they are needed, often quite vigorously. They divide in two like bacteria, with apparent independence, and even fuse together into great branching networks. Mitochondria were first detected using light microscopy, as granules, rods, and filaments in the cell, but their provenance was debated from the beginning. Among the first to recognize their importance was the German Richard Altmann, who argued that the tiny granules were in truth the fundamental particles of life, and accordingly named them
bioblasts
in 1886. For Altmann, the bioblasts were the only living components of the cell, which he held to be little more than a fortified community of bioblasts living together for mutual protection, like the people of an iron-age fortification. Other structures, such as the cell membrane and the nucleus, were constructed by the community of bioblasts for their own ends, while the cytosol (the watery part of cytoplasm), was just that: a reservoir of nutrients enclosed in the microscopic fortress.
Altmann’s ideas never caught on, and he was ridiculed by some. Others claimed that bioblasts were a figment of his imagination—merely artefacts
of his elaborate microscopic preparation. These disputes were aggravated by the fact that cytologists had become entranced by the stately dance of the chromosomes during cell division. To visualize this dance, the transparent components of the cell had to be coloured using a stain. As it happened, the stains that were best able to colour the chromosomes were acidic. Unfortunately, these stains tended to dissolve the mitochondria; their obsession with the nucleus meant that cytologists were simply dissolving the evidence. Other stains were ambivalent, colouring mitochondria only transiently, for the mitochondria themselves rendered the stain colourless. Their rather ghostly appearance and disappearance was scarcely conducive to firm belief. Finally Carl Benda demonstrated, in 1897, that mitochondria do have a corporeal existence in cells. He defined them as ‘granules, rods, or filaments in the cytoplasm of nearly all cells … which are destroyed by acids or fat solvents.’ His term,
mitochondria
(pronounced ‘my-toe-con-dree-uh’), was derived from the Greek
mitos
, meaning thread, and
chondrin
, meaning small grain. Although his name alone stood the test of time, it was then but one among many. Mitochondria have revelled in more than thirty magnificently obscure names, including chondriosomes, chromidia, chondriokonts, eclectosomes, histomeres, microsomes, plastosomes, polioplasma, and vibrioden.
While the real existence of mitochondria was at last ceded, their function remained unknown. Few ascribed to them the elementary life-building properties claimed by Altmann; a more circumscribed role was sought. Some considered mitochondria to be the centre of protein or fat synthesis; others thought they were the residence of genes. In fact, the ghostly disappearance of mitochondrial stains finally gave the game away: the stains were rendered colourless because they had been
oxidized
by the mitochondria—a process analogous to the oxidation of food in cell respiration. Accordingly, in 1912, B. F. Kingbury proposed that mitochondria might be the respiratory centres of the cell. His suggestion was demonstrated to be correct only in 1949, when Eugene Kennedy and Albert Lehninger showed that the respiratory enzymes were indeed located in the mitochondria.
Though Altmann’s ideas about bioblasts fell into disrepute, a number of other researchers also argued that mitochondria were independent entities related to bacteria,
symbionts
that lived in the cell for mutual advantage. A symbiont is a partner in a symbiosis, a relationship in which both partners benefit in some way from the presence of the other. The classic example is the Egyptian plover, which picks the teeth of Nile crocodiles, providing dental hygiene for the crocodile while gaining an easy lunch for itself. Similar mutual relationships can exist among cells such as bacteria, which sometimes live inside larger cells as
endosymbionts
. In the first decades of the twentieth century, virtually all parts of the cell were considered as possible endosymbionts, perhaps modified
by their mutual coexistence, including the nucleus, the mitochondria, the chloroplasts (responsible for photosynthesis in plants), and the centrioles (the cell bodies that organize the cytoskeleton). All these theories were based on appearance and behaviour, like movement and apparently autonomous division, and so could never be more than suggestive. What’s more, their protagonists were all too often divided by struggles over priority, by war and language, and rarely agreed among themselves. As the science historian Jan Sapp put it, in his fine book
Evolution by Association
: ‘Thus unfolds an ironic tale of the fierce individualism of many personalities who pointed to the creative power of associations in evolutionary change.’
Matters came to a head after 1918, when the French scientist Paul Portier published his rhetorical masterpiece
Les Symbiotes
. He was nothing if not bold, claiming that: ‘All living beings, all animals from Amoeba to Man, all plants from Cryptogams to Dicotyledons are constituted by an association, the
emboîtement
of two different beings. Each living cell contains in its protoplasm formations, which histologists designate by the name of mitochondria. These organelles are, for me, nothing other than symbiotic bacteria, which I call symbiotes.’
Portier’s work attracted high praise and harsh criticism in France, though it was largely ignored in the English-speaking world. For the first time, however, the case did not stand on the morphological similarities between mitochondria and bacteria, but turned on attempts to cultivate mitochondria as a cell culture. Portier claimed to have done so, at least with ‘proto-mitochondria’, which he argued had not yet become fully adapted to their life inside cells. His findings were publicly contested by a panel of bacteriologists at the Pasteur Institute, who were unable to replicate them. And sadly, once he had secured his chair at the Sorbonne, Portier abandoned the field, and his work was quietly forgotten.
A few years later, in 1925, the American Ivan Wallin independently put forward his own ideas on the bacterial nature of mitochondria, claiming that such intimate symbioses were the driving force behind the origin of new species. His arguments again turned on culturing mitochondria, and he, too, believed that he had succeeded. But for a second time interest waned with the failure to replicate his work. This time symbiosis was not ruled out with quite the same venom, but the American cell biologist E. B. Wilson summed up the prevailing attitude in his famous remark: ‘To many, no doubt, such speculations may appear too fantastic for present mention in polite biological society; nevertheless it is within the range of possibility that they may some day call for some serious consideration.’
That day turned out to be half a century later: aptly enough for the tale of an intimate symbiotic union, in the summer of love. In June 1967, Lynn Margulis submitted her famous paper to the
Journal of Theoretical Biology
, in which she
resurrected the ‘entertaining fantasies’ of previous generations and cloaked them in newly scientific apparel. By then the case was much stronger: the existence of DNA and RNA in mitochondria had been proved, and examples of ‘cytoplasmic heredity’ catalogued (in which inherited traits were shown to be independent of the nuclear genes). Margulis was then married to the cosmologist Carl Sagan, and she took a similarly cosmic view of the evolution of life, considering not just the biology, but also the geological evidence of atmospheric evolution, and fossils of bacteria and early eukaryotes. She brought to the task a consummate discernment of microbial anatomy and chemistry, and applied systematic criteria to determine the likelihood of symbiosis. Even so, her work was rejected. Her seminal paper was turned down by 15 different journals before James Danielli, the far-seeing editor of the
Journal of Theoretical Biology
, finally accepted it. Once published, there were an unprecedented 800 reprint requests for the paper within a year. Her book,
The Origin of Eukaryotic Cells
, was rejected by Academic Press, despite having been written to contract, and was eventually published by Yale University Press in 1970. It was to become one of the most influential biological texts of the century. Margulis marshalled the evidence so convincingly that biologists now accept her once-heterodox view as fact, at least when applied to mitochondria and chloroplasts.
Bitter arguments persisted for well over a decade, and were arcane but vital. Without them, the final agreement would have been less secure. Everyone accepted that there are indeed parallels between mitochondria and bacteria, but not everyone agreed about what these really meant. Certainly the mitochondrial genes are bacterial in nature: they sit on a single circular chromosome (unlike the linear chromosomes of the nucleus) and are ‘naked’—they’re not wrapped up in histone proteins. Likewise, the transcription and translation of DNA into proteins is similar in bacteria and mitochondria. The physical assembly of proteins is also managed along similar lines, and differs in many details from standard eukaryotic practice. Mitochondria even have their own ribosomes, the protein-building factories, which are bacterial in appearance. Various antibiotics work by blocking protein assembly in bacteria, and also block protein synthesis in the mitochondria, but not from the nuclear genes in eukaryotes.
Taken together, these parallels might sound compelling, but in fact there are possible alternative interpretations, and it was these that underpinned the long dispute. In essence, the bacterial properties of mitochondria could be explained if the speed of evolution was slower in the mitochondria than in the nucleus. If so, then the mitochondria would have more in common with bacteria simply because they had not evolved as fast, and so as far. They would retain more atavistic traits. Because the mitochondrial genes are not recombined by sex, this position was sustainable, if somewhat unsatisfying. It could
only be refuted when the actual rate of evolution was known, which in turn required the direct sequencing of mitochondrial genes, and the comparison of sequences. Only after Fred Sanger’s group in Cambridge had sequenced the human mitochondrial genome in 1981 did it transpire that the evolution rate of mitochondrial genes was
faster
than that of the nuclear genes. Their atavistic properties could only be explained by a direct relationship; and this relationship was ultimately shown to be with a very specific group of bacteria, the α-proteobacteria.