The Cerebellum: Brain for an Implicit Self (25 page)
Read The Cerebellum: Brain for an Implicit Self Online
Authors: Masao Ito
Tags: #Science, #Life Sciences, #Medical, #Biology, #Neurology, #Neuroscience

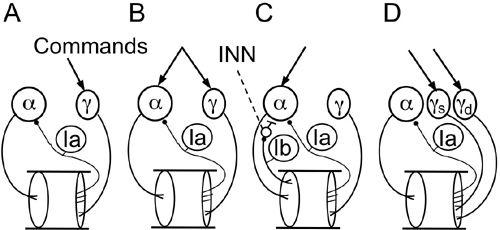
Figure 31. Hypotheses on key neural control mechanisms for muscle activation.
Sketches show four hypotheses of particular relevance to this chapter. (A) Length follow-up servo. Commands first activate γ-motoneurons, followed by reflex activation of α-motoneurons. (B) Coactivation of both α- and γ-motoneurons for length servo-assisted motion. (C) Negative force feedback from tendon organs and negative length feedback from muscle spindles cooperating to stabilize muscle stiffness. INN, inhibitory interneuron. (D) Fusimotor set of static (γ s) and dynamic (γ d) motoneurons. For further information, see the text. (Based on ideas of
Loeb, 1984
;
Nichols and Ross, 2009
;
Prochazka and Ellaway, 2011
.)
Group Ib afferents, on the other hand, arise from Golgi tendon organs that sense the force of muscle contraction and, within the spinal cord, their impulse signals may, under some conditions, inhibit α-motoneurons via inhibitory interneurons. Hence, this type of Ib stretch reflex (with short and long latency components) may provide a negative feedback control (force feedback; see
Nichols and Ross, 2009
), which can cooperate with the Ia stretch reflex to regulate muscle stiffness (
Figure 31C
). However, whereas this control occurs during “resting” states in spinal and decerebrate cats, and several other species, the reflex effects of Ib afferents switch to excitatory during terrestrial locomotion (
Pearson and Collins, 1993
).
This results in group Ib afferents providing positive force feedback, which strengthens the stance phase of the step (for further details, see
Prochazka et al., 1997
;
Hultborn, 2001
;
Nichols and Ross, 2009
).
Based on these observations on Ia and Ib afferents, four hypotheses have been proposed to explain different, largely spinal neural control mechanisms for muscle contraction.
Figure 31A
shows the classical length follow-up servo, in which γ-motoneurons play the role of initiating muscle contraction; a change in their firing rates will cause a similar reflex-induced change in the activation level of extrafusal muscle fibers (
Merton, 1953
). The second hypothesis shown in
Figure 31B
is length-servo-assisted motion, in which α- and γ-motoneurons are coactivated by the same command signals (
Granit, 1975
). This process prevents the length-servo from resisting muscle contraction. The third hypothesis shown in
Figure 31C
involves negative force feedback from tendon organs and negative length feedback from muscle spindles cooperating to stabilize the stiffness of the muscle (
Houk, 1979
). As mentioned previously, this Ib effect changes to positive feedback during locomotion, thus assisting in the stance phase of the step. The fourth hypothesis of “fusimotor set” shown in
Figure 31D
is based on the finding that γ-motoneurons have two subtypes; static and dynamic. Activity of dynamic γ-motoneurons is enhanced in certain occasions when spindle endings may fire at very high rates during large fluctuations in muscle length (
Prochazka et al., 1985
). Fusimotor set may also be adjusted to optimize spindle sensitivity according to anticipated variations in kinematics for particular movements (
Loeb et al., 1990
). Each of these four hypotheses accounts for only a highly constrained class of muscle functions, and a general theory is still awaited (see
Loeb, 1984
;
Prochazka and Ellaway, 2011
).
Spindle group II afferents arise from an area off the middle of muscle spindles. In the spinal cord, they make mono-, di-, and trisynaptic connections with α-motoneurons. In the spinal cord, they may excite or inhibit flexor muscles and do the reverse to extensor muscles, depending on the nature and context of the movement. For example, it has been shown in a study on human treadmill locomotion that when the soleus muscle was stretched by applying small amplitude dorsiflexion perturbations shortly after heel contact, short and medium latency responses were observed. The short latency response was velocity sensitive, whereas the medium latency one was not. The medium latency component was more sensitive than the short latency one to nerve cooling, but more resistant to ischemia. Two hours after the ingestion of tizanidine, an α
2
-adrenergic receptor agonist known to selectively depress transmission in the group II muscle afferent pathway, the
medium latency reflex was strongly depressed, whereas the short latency component was unchanged. Based on these observations, it was suggested that the medium latency component of the stretch reflex represented responses of spindle II afferents induced by an unexpected perturbation (
Grey et al., 2001
). A general theory on spindle afferent II function is also yet to emerge.
Myotatic reflexes.
Each joint is attached by a group of agonist muscles on one side and another group of antagonist muscles on the other (recall
Figure 3A
). As stated previously, the short-latency part of the stretch reflex in a muscle evokes group-Ia afferent signals, which excite α-motoneurons of that muscle and its agonists. At the same time, the group-Ia afferent signals excite Ia inhibitory neurons mediating “Ia reciprocal inhibition” of α-motoneurons supplying antagonist muscles. In this way, the short-latency stretch reflex in a muscle is sometimes accompanied by the contraction of agonist muscles and the relaxation of antagonist muscles. Each joint is equipped with a pair of such stretch reflex-Ia inhibition combinations, this being called a myotatic reflex (
Figure 32
). In each joint of a limb, myotatic reflexes determine under some conditions the position of the joint by balancing two muscle groups—that is, extensor muscles acting to extend the limb and flexor muscles acting to flex the limb.
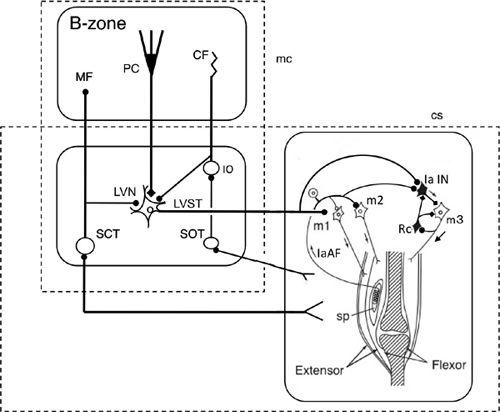
Figure 32. Wiring diagram for cerebellar control of the short-latency (tendon-tap) myotatic reflex.
Some CNS connections are shown for a pair of agonist (extensor) and antagonist (flexor) muscles when the agonist is exhibiting a tendon-tap reflex (correction) brought on by a perturbation. The right box shows that muscle spindles (sp) in the agonist activate their own motoneurons (m1 and m2) via Ia afferents (IaAF) and inhibit their antagonist motoneurons (m3) via Ia inhibitory interneurons (IaIN). The circuitry also includes Renshaw cells (Rc), which exert recurrent inhibition in the m3 motoneurons. This segmental circuit is controlled by Deiters neurons via the lateral vestibular nucleus (LVN) and the lateral vestibulospinal tract (LVST). The LVN is modulated by the B zone of the cerebellum, which receives proprioceptive and exteroceptive information via the dorsal and ventral spinocerebellar tracts (SCT). The B-zone and LVN forn a microcomplex, which serves as an adaptive controller for stretch reflexes. Proprioceptive information from muscles and tendons as well as cutaneous signals evoked by a perturbation of posture are transferred to the cerebellum via the spinoolivary tract (SOT). Additional abbreviations: CF, climbing fibers; MF, mossy fibers; PC, Purkinje cells.
Feldman’s equilibrium point hypothesis (
1986
) proposed that the position of each joint is set by the equilibrium of the spring-like tension-length relationship between two antagonistic muscles crossing the joint. It may further be assumed that, involving both the length- and force-feedback pathways from muscle spindles and Golgi tendon organs, respectively, the force-length relationship for each muscle will be determined by control signals from the CNS. The set of the equilibrium points so determined for the agonist-antagonist pairs of muscles crossing a joint represents the position of the joint, and changes in patterns of these equilibrium points in time would represent trajectories leading a movement (for a recent evaluation of Feldman’s hypothesis, see
Nichols and Ross, 2009
).
Descending control.
The functional pattern of excitatory inputs from several descending pathways (see following description) and sensory input received by motoneurons that supply one set of muscles appears to be replicated onto the Ia inhibitory interneurons projecting to the motoneurons that supply the muscles that are antagonist to the homonymous set. This similarity in the convergence onto “corresponding” motoneurons and Ia inhibitory interneurons led Lundberg and his colleagues to hypothesize that both motoneurons and Ia inhibitory interneurons are activated in parallel during voluntary movements to secure a coordinated contraction of agonists and relaxation of antagonists (
Hongo et al., 1969
; Lundberg, 1970).
Myotatic reflexes and spinal pattern generators are modulated by several descending pathways including the cortico-, reticulo-, rubro- (weak in humans), and
vestibulo-spinal descending tracts. Among these, descending signals of the lateral vestibulospinal tract facilitate extensor motoneurons supplying hindlimb, forelimb, and neck motoneurons (
Figure 32
). (Note, however, that the monosynaptic linkage between vestibulospinal fibers and motoneurons is mainly with respect to neck motoneurons;
Wilson and Yoshida, 1969
.) Signals of the lateral vestibulospinal fibers also excite the Ia inhibitory interneurons responsible for Ia reciprocal
inhibition from extensors to flexors (
Grillner et al., 1971
). In addition to these connections, various effects in terms of excitation/inhibition and latency were induced in spinal motoneurons by stimulating reticulospinal tracts (
Peterson et al., 1979
). Stimulation of the rubrospinal tract also induced excitation of flexor and inhibition of extensor motoneurons, but a mixture of EPSPs and IPSPs was found in many motoneurons (
Hongo et al., 1969
). These effects were mediated by segmental interneurons. It has also been shown that the lateral vestibulospinal tract and part of the reticulospinal tract project to contralateral motor nuclei via common commissural inhibitory interneurons (
Krutki et al., 2003
).
It has recently been reported that locally injected orexin A excites lateral vestibular nuclear neurons and improves vestibular-related motor behavior in rats, including vestibular-mediated posture, motor balance, and negative geotaxis. To estimate the negative geotaxis, rats were placed on a 40° incline with their head pointing down the slope, and the time spent for a turn of 180° upward against the direction of the gravitational field was measured. The motor effects of orexin A were prominent when an animal faced a major motor challenge such as when rats traversed an inclined balance beam or stayed on an accelerating rota-rod, as compared to during rest and the execution of everyday movements (
Zhang et al., 2011
). Note also that orexins are also involved in defense reactions (
Section 6
).
Cerebellar function.
The possibility that stretch reflexes operate under the influence of the cerebellum via spinal descending tracts has been examined by recording a short-latency stretch reflex in a muscle stimulated by sinusoidal stretch at 0.1–10 Hz. The stretch reflex so evoked displayed a phase advance relative to the stretch stimuli. This advance was reduced by acute and chronic cerebellectomy (decerebrate cat;
Higgins, 1987
). Apparently, this effect reflects the contribution of the cerebellum to improve dynamic characteristics of the short-latency stretch reflex. In the dorsal area of the lateral vestibular nucleus of Deiters, neurons of origin of lateral vestibulospinal tract fibers receive Purkinje cell inhibition directly from the B-zone of the cerebellum (
Figure 32
). These dorsal Deiters neurons also receive excitatory synapses from spinal ascending tracts (
Akaike et al., 1973
). This shows that Deiters neurons mediate a supraspinal loop superposed on a segmental stretch reflex, and thereby mediate an adaptive action from the B-zone as illustrated in
Figure 32
. Stretch reflexes may also be under the influence of the cerebellar A-zone, which, via the fastigial nucleus, regulates adaptively pontine reticulospinal tract axons (
Ito et al., 1970
).