Authors: George M. Church
Regenesis (8 page)
A cell's membrane, which constitutes its outer surface, is composed of lipid molecules. Lipid molecules possess special properties that suit them to the cell's watery interior and to its fluid external environment. A lipid molecule is a long linear structure whose two ends behave differently when immersed in water, which in the case of living cells is most of the time. One end of the molecule, the “head,” carries an electrical charge, and this makes that end hydrophilic (water-loving), meaning that it seeks out or orients itself toward water. The molecule's other end, the “tail,” is uncharged, which makes it hydrophobic (water-fearing), meaning that it hides or sequesters itself from water. This bipolar feature makes lipid molecules into active, dynamic entities, for when placed in water they will spontaneously associate and collectively adopt the shape suited to the opposed chemical affinities of their ends: generally they will form a sphere, with the outer surface consisting of the charged heads, the inner surface of uncharged tails. Cell membranes are composed of a lipid bilayer, two sheets of lipid molecules sandwiched together.
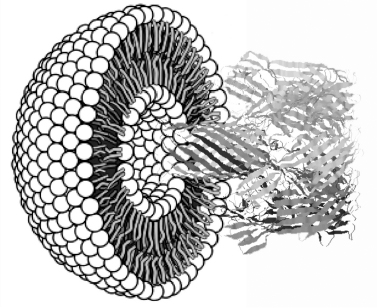
Figure 2.1
Lipid bilayer spherical shell (cross-section) with a protein pore (hemolysin) on right showing how molecules can cross the otherwise impermeable bilayer.
Collectively, these three cellular elementsânucleic acids, proteins, and the lipid bilayer membraneâexist for the purpose of maintaining the cell
as a living system, but for this it needs a fourth class of materials, sugars (saccharides, typically glucose or sucrose). Sugars are the material sources of a cell's energy. But in order to be actually utilized as energy, they must first be converted into a biochemically accessible form. This conversion occurs in a process called glycolysis (“sugar breaking”), a complex, ten-step progression of events that is the bane of biochemistry students (although other metabolic pathways take even more twists and turns). The final result of glycolysis is the creation of ATP (adenosine triphosphate), the molecules that directly power the cell. Those same ATP molecules power our bodily cells.
To be a cell, then, is to be a deterministic system governed by DNA, composed largely of proteins and lipids, and energized by ATP. Some cells are
more like us than you may imagine
â
E. coli
, for example, the standard organism of genetic engineering.
This bacterium was discovered in 1885 by the German pediatrician Theodor Escherich, after whom it was named. It has since become the most biochemically well-defined organism known to science. Like ourselves, it is mobile and self-propelled, although the medium through which it travels and its propulsion system are quite different from our own. Individual
E. coli
cells are small, rod-shaped objects about four micrometers in length, easily visible in a light microscope. Extending from the cell's surface are a number of long, corkscrew-shaped flagella. They propel the cell through a watery medium that, to them, is as viscous as molasses. At this scale, where gravity has little effect, there is no up or down. Since the average
E. coli
cell lives inside the human intestinal tract, the cell has no vision, and since it has no brain or nervous system, it has no conscious experience. But believe it or not, the bacterium has a primitive sense of perception.
An
E. coli
cell can be observed swimming through its aqueous medium in a straight line, propelled by its flagella motors. Inside the cell, floating in the cytoplasm, certain kinds of protein molecules react to the density of nutrients in the surrounding medium. If the nutrient levels remain the same or are increasing, the bacterium will continue straight ahead in its travels. But if the nutrient level declines, these same proteins will react with the flagellar motor in such a way that causes the organism to reverse direction. It will then flail about and wander all over, as if exploring the universe, until the nutrient gradient increases, whereupon it will again swim in the direction of the nutrient. In its simple and rudimentary way, this is how the bacterium senses its surroundings.
There is, finally, one other commonality between ourselves and
E. coli
, and that is the ability to reproduce. An
E. coli
cell's capacity for reproduction is unmatched in speed by few other organisms. The basic processes take place in the cytoplasm, which has no precise analog on the human scale. It's a scene of apparent pandemonium where every possible space is filled, all molecules are in a state of constant thermal agitation, and objects of multiple shapes and sizes are bumping into each other, fusing together, breaking apart, and popping into and out of existence seemingly at random.
Biochemist David Goodsell, in his book
The Machinery of Life
, likens the conditions inside the cytoplasm to an airport terminal crowded with passengers who are pushing, shoving, and scrambling in all directions. But this image doesn't really do justice to the interior of an
E. coli
cell. An airport terminal, after all, is a rigid and stable structure that exists in two dimensions (or if we take into account the stratification of arriving and departing as well as domestic and international passengers, and so on, we could attribute to it a slightly larger fractional dimensionality such as 2.1), whereas the cytoplasm is unquestionably a dense three-dimensional throbbing blob. Inside it, however, everything careens relentlessly toward self-replication, for within the space of about thirty minutes, the cell manages to duplicate with extreme precision each and every one of its component parts: its proteins, lipid molecules, even its own genome. And at the end of the process, the cell pinches itself in two, giving birth to a daughter cell clone, which will reproduce itself in another half hour.
The role of
E. coli
in genetic engineering stems from its ability to reproduce itself with its characteristic high speed and great fidelity. According to a standard account (which is probably correct), genetic engineering in the modern sense was born in 1972, when two biologists met for a late-night snack at a delicatessen near Waikiki beach in Hawaii. Stanford University medical professor Stanley Cohen and biochemist Herbert Boyer, of the University of CaliforniaâSan Francisco, were in Honolulu to attend a conference on plasmids, the circular strands of DNA found in the cytoplasm of bacteria. Plasmids can be replicated independently of the cell's chromosomes.
At the conference, Cohen announced that he could insert plasmid DNA into
E. coli
and have the bacterium propagate and clone the plasmid. Boyer described his work with EcoRI, an enzyme (named after and isolated from
E. coli
) that could cut DNA at specific, predefined sites along the length of the molecule. Later that night the two scientists realized that by combining their respective innovations they could splice together fragments of two different plasmids, producing recombinant (mechanically changed) DNA, and then get the bacterium to mass-produce whatever it was that the engineered plasmid coded for.
But as correct as that account may be, Cohen and Boyer were not the world's first (nor even the most successful) genetic engineers. That distinction belongs to viruses, particularly bacteriophages (such as the T4 phage, which looks like a lunar landing module straight from the Apollo program).
A virus is essentially a string of DNA or RNA wrapped in a protein coating. It replicates by inserting its genome into a fully fledged cell, which proceeds to treat this new and foreign set of raw genes as if it were its own original genetic material. Uninfected cells use nucleic acids primarily to make proteins: a molecule of RNA polymerase (an enzyme) unzips a section of double-stranded DNA, reads off its genetic information, and constructs a complementary strand of mRNA (messenger RNA), in a process called transcription. The mRNA is in turn read by ribosomes, along with some other molecular machines, which collectively string together amino acids in the order prescribed by the mRNA, in a process called translation. Since a protein is nothing but a long string of amino acids, when the translation is complete, so is the protein.
Infection by a virus changes the picture entirely. For an
E. coli
bacterium, an attack by a phage virus initiates a cascade of violence and destruction equal to anything offered up by horror fiction. The phages descend on the bacterium's outer membrane like a swarm of bees and forcefully insert their DNA through the cell membrane and into the cytoplasm. The viral DNA invaders now sabotage the cell's normal transcription and translation of the its own genes, and redirect those processes on themselves. At that point, the viral genome has taken over the organism, and some minutes later, with its own enzymes firmly in command of the cell, the virus has been replicated tens to hundreds of times.
The final stepâlysis, “bursting”âis the climax of the process.
Under the Microscope
, a collection of photographs taken with electron microscopes, graphically depicts the very moment of the cell's destruction: a malign-looking fleet of new T4 phage particles, each of them an exact duplicate of the lunar landing module virus that started the whole chain reaction, pour out of the bacterium's ruptured membrane in what looks like a microbial-level explosion.
This is genetic engineering as done by nature.
This process is not so different from the way it's done in the lab. As pioneered by Cohen and Boyer, who patented their procedure in 1976, and as practiced by hordes of genetic engineers who followed them, from the ranks of biotech companies to high school students, a gene of interest is inserted into a colony of
E. coli
bacteria, often by means of a plasmid containing the stretch of DNA that codes for the desired substance. To the bacterium, a gene is a gene. So long as the newly inserted DNA segment makes molecular-biological sense (which in many cases it doesn't), the bacterium will produce whatever the gene codes for, and in generally the same way that it manufactures viruses.
Getting
E. coli
to churn out jet fuel is nothing compared to T4 phage viruses getting the same bacterium to fabricate more of themselves; the phages are far more complex entities than simple kerosene molecules.
E. coli
bacteria are tiny, obedient molecular factories. You tell them what to make and, by and large, they will make it.
Now we know what it's like to be a cell, particularly an
E. coli
cell, and we understand why it's the vehicle of choice for genetic and genomic engineering. In the Prologue, we saw that with Fred Blattner's Clean Genome
E. coli
, it's possible to improve on and streamline the bacterium's native genetic software. In nature,
E. coli
has 4,377 genes on a genome consisting of 4,639,221 base pairs. Blattner reduced the gene count of the laboratory K-12 strain by some 15 percent, thereby producing an organism that was optimized for laboratory, industrial, and academic research purposes. Within limits, organisms are plastic and malleable, and are susceptible to improvements and efficiencies through intelligent engineering. Indeed, it is a central tenet of synthetic biology that organisms as we find them are not necessarily optimized for accomplishing the various specific tasks to which we might put them.
