The Washington Manual Internship Survival Guide (15 page)
Read The Washington Manual Internship Survival Guide Online
Authors: Thomas M. de Fer,Eric Knoche,Gina Larossa,Heather Sateia
Tags: #Medical, #Internal Medicine

•
Acidemia
(pH <7.37) results from either decreased [HCO
3
-
] or increased PCO
2
.
•
Alkalemia
(pH >7.43) results from either increased [HCO
3
-
] or decreased PCO
2
.
•
An ABG, electrolyte panel, and a serum [HCO
3
-
] are required to assess acid/base status.
•
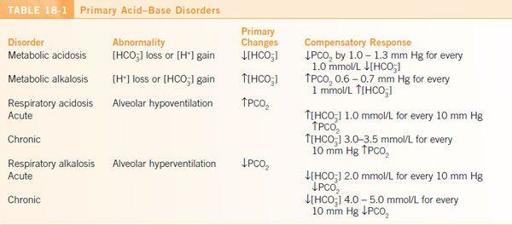
Stepwise approach to an ABG:
1.
Examine the pH. Is the patient acidemic or alkalemic?
2.
Establish the primary disturbance.
a.
Examine the [HCO
3
-
]. In primary metabolic disorders, it moves in the same direction as the pH.
b.
Examine the PCO
2
. In primary respiratory disorders, it moves in the opposite direction as the pH.
c.
A combined disorder is present when 1) pH is normal but PCO
2
and [HCO
3
-
] are both abnormal or 2) changes in both PCO
2
and [HCO
3
-
] can cause the change in pH.
3.
Is there adequate respiratory or metabolic compensation? If there is not adequate compensation, there may be a combined disorder present (
Table 18-1
).
4.
If a metabolic acidosis is present:
a.
Calculate the anion gap: AG = [Na
+
] - ([Cl
−
] + [HCO
3
-
]).
b.
If no gap is present, calculate the urine anion gap: UAG = U
[Na+]
+ U
[K+]
- U
[Cl-]
. A negative UAG suggests GI HCO
3
-
losses, whereas a positive UAG suggests an RTA.
5.
If there is an anion gap, assess the delta gap:
a.
AG
correct
= AG + {(4 - [albumin]) × 2.5}
b.
ΔAG = AG
correct
- 10
c.
Δ[HCO
3
] = 24 - [HCO
3
]
d.
ΔAG = Δ[HCO
3
-
] indicates simple AG metabolic acidosis.
e.
ΔAG >Δ[HCO
3
-
] indicates AG metabolic acidosis and metabolic alkalosis.
f.
ΔAG <Δ[HCO
3
-
] indicates AG metabolic acidosis and nongap metabolic acidosis.
METABOLIC ACIDOSIS
Etiology and Diagnosis
See
Table 18-2
.
Treatment
•
Treatment of the underlying condition should be the primary focus.
•
Severe acidosis (pH <7.20) may require treatment with parenteral NaHCO
3
. Rapid infusion should be considered only for very severe acidosis.

•
Overaggressive correction should be avoided to prevent overshoot alkalosis.
•
Adverse effects of parenteral NaHCO
3
include pulmonary edema, hypernatremia, hypokalemia, and hypocalcemia. Monitor electrolytes frequently.
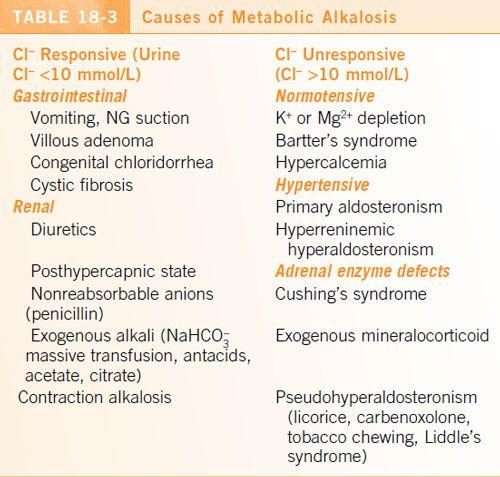
METABOLIC ALKALOSIS
Etiology
•
Metabolic alkalosis may be caused by HCO
3
-
gain, H
+
loss, or volume contraction.
•
Vomiting and diuretic use are the two most common causes.
•
See
Table 18-3
.
Treatment
•
Treatment of the underlying condition should be the primary focus.
•
Correct hypokalemia and hypomagnesemia.
•
Chloride-responsive metabolic alkaloses should be treated with isotonic NS
.
•
Chloride-unresponsive metabolic alkaloses do not improve with saline administration.
• K
+
-sparing diuretics (e.g., amiloride, spironolactone) are effective for mineralocorticoid excess.
• In patients with normal renal function, alkalosis from excessive alkali administration will resolve quickly once the HCO
3
-
load is withdrawn.
•
Acetazolamide may be useful if alkalosis persists despite the above interventions or if saline administration is limited by volume overload.
RESPIRATORY ACIDOSIS
Etiology
•
↑PCO
2
is almost always the result of alveolar hypoventilation.
•
In
acute respiratory acidosis
, the pH ↓0.08 for every 10 mm Hg ↑PCO
2
above 40 mm Hg.
•
In
chronic respiratory acidosis
, the pH ↓0.03 for every 10 mm Hg ↑PCO
2
above 40 mm Hg.
•
Renal compensation takes several days to develop fully.
•
See
Table 18-4
.
Treatment
•
Treatment is directed at the underlying condition.
•
Potentially contributing drugs should be stopped or counteracted (e.g., naloxone, flumazenil).


•
Ventilatory assistance may be required (CPAP, BiPAP, or mechanical ventilation).
•
Avoid NaHCO
3
administration as this can worsen hypercapnia (HCO
3
-
combines with H
+
in the tissues to form CO
2
+ H
2
O).
RESPIRATORY ALKALOSIS
Etiology
•
It is important to remember that tachypnea/hyperventilation does not necessarily imply a simple respiratory alkalosis. If you have any uncertainty, obtain an ABG.
•
See
Table 18-5
.
Treatment
•
Treatment is directed at the underlying condition.
•
Psychogenic hyperventilation may be treated by rebreathing from a paper bag.
ECG and Radiography
19
ECG
TACHYCARDIA
•
Tachyarrhythmias are broadly categorized as wide-complex tachycardia (WCT) and narrow-complex tachycardia (NCT).
•
In the hemodynamically unstable patient with a tachyarrhythmia, immediately ask for help, initiate ACLS if warranted, and place defibrillator pads to prepare for electrical cardioversion.
•
In the otherwise stable patient, some time and thought can lead to a satisfying diagnosis!
Narrow-Complex Tachycardias
•
NCTs are almost
always supraventricular in origin
. When dealing with NCTs it is useful to first assess
whether the rhythm is regular or irregular
(
Figure 19-1
).
•
If the tachyarrhythmia is regular, a standard 12-lead ECG should be examined for p-waves. The rhythm can then be further categorized by assessing whether the p-wave is closer to the R-wave that precedes it (
short R-P tachycardia
) or the R-wave that follows it (
long R-P tachycardia
).