Penny le Couteur & Jay Burreson (2 page)
Read Penny le Couteur & Jay Burreson Online
Authors: Napoleon's Buttons: How 17 Molecules Changed History
Tags: #Philosophy & Social Aspects, #Science, #General, #World, #Chemistry, #Popular Works, #History

The choice of which compounds to include in this book was a personal one, and the final selection is by no means exhaustive. We have chosen those compounds we found the most interesting for both their stories and their chemistry. Whether the molecules we selected are definitely the most important in world history is arguable; our colleagues in the chemical profession would no doubt add other molecules to the list or remove some of the ones we discuss. We will explain why we believe certain molecules were the impetus for geographic exploration, while others made possible the ensuing voyages of discovery. We will describe molecules that were critical to the development of trade and commerce, that were responsible for human migrations and colonization, and that led to slavery and forced labor. We will discuss how the chemical structure of some molecules has changed what we eat, what we drink, and what we wear. We will look at molecules that spurred advances in medicine, in public sanitation, and in health. We will consider molecules that have resulted in great feats of engineering, and molecules of war and peaceâsome responsible for millions of deaths while others saving millions of lives. We will explore how many changes in gender roles, in human cultures and society, in law, and in the environment can be attributed to the chemical structures of a small number of crucial molecules. (The seventeen molecules we have chosen to focus on in these chaptersâthe seventeen molecules referred to in the titleâare not always individual molecules. Often they will be groups of molecules with very similar structures, properties, and roles in history.)
The events discussed in this book are not arranged in chronological historical order. Instead, we have based our chapters on connectionsâthe links between similar molecules, between sets of similar molecules, and even between molecules that are quite different chemically but have properties that are similar or can be connected to similar events. For example, the Industrial Revolution owes its start to the profits reaped from a slave-grown compound (sugar) on plantations in the Americas, but it was another compound (cotton) that fueled major economic and social changes in Englandâand chemically the latter compound is a big brother, or maybe a cousin, of the former compound. The late-nineteenth-century growth of the German chemical industry was due, in part, to the development of new dyes that came from coal tar (a waste material arising from the production of gas from coal). These same German chemical companies were the first to develop man-made antibiotics, composed of molecules with similar chemical structures to the new dyes. Coal tar also provided the first antiseptic, phenol, a molecule that was later used in the first artificial plastic and is chemically related to isoeugenol, the aromatic molecule from nutmeg. Such chemical connections are abundant in history.
We were also intrigued by the role serendipity has been accorded in numerous chemical discoveries. Luck has often been cited as crucial to many important findings, but it seems to us that the ability of the discoverers to realize that something unusual has happenedâand to question why it occurred and how it could be usefulâis of greater importance. In many instances in the course of chemical experimentation an odd but potentially important result was ignored and an opportunity lost. The ability to recognize the possibilities in an unexpected result deserves to be lauded rather than dismissed as a fortuitous fluke. Some of the inventors and discoverers of the compounds we discuss were chemists, but others had no scientific training at all. Many of them could be described as charactersâunusual, driven, or compulsive. Their stories are fascinating.
ORGANIC-ISN'T THAT GARDENING?To help you understand the chemical connections in the following pages, we'll first provide a brief overview of chemical terms. Many of the compounds discussed in this book are classified as
organic
compounds. During the last twenty or thirty years the word organic has taken on a meaning quite different from its original definition. Nowadays the term
organic,
usually in reference to gardening or food, is taken to mean agriculture conducted without artificial pesticides or herbicides and with no synthetic fertilizers. But
organic
was originally a chemical term dating back nearly two hundred years to Jöns Jakob Berzelius, a Swedish chemist who in 1807 applied the word organic to compounds that were derived from living organisms. In contrast, he used the word
inorganic
to mean compounds that did not come from living things.
organic
compounds. During the last twenty or thirty years the word organic has taken on a meaning quite different from its original definition. Nowadays the term
organic,
usually in reference to gardening or food, is taken to mean agriculture conducted without artificial pesticides or herbicides and with no synthetic fertilizers. But
organic
was originally a chemical term dating back nearly two hundred years to Jöns Jakob Berzelius, a Swedish chemist who in 1807 applied the word organic to compounds that were derived from living organisms. In contrast, he used the word
inorganic
to mean compounds that did not come from living things.
The idea that chemical compounds obtained from nature were somehow special, that they contained an essence of life even though it could not be detected or measured, had been around since the eighteenth century. This special essence was known as
vital energy.
The belief that there was something mystical about compounds derived from plants or animals was called
vitalism.
Making an organic compound in the laboratory was thought to be impossible by definition, but ironically one of Berzelius's own students did just that. In 1828, Friedrich Wöhler, later professor of chemistry at the University of Göttingen in Germany, heated the inorganic compound ammonia with cyanic acid to produce crystals of urea that were exactly the same as the organic compound urea isolated from animal urine.
vital energy.
The belief that there was something mystical about compounds derived from plants or animals was called
vitalism.
Making an organic compound in the laboratory was thought to be impossible by definition, but ironically one of Berzelius's own students did just that. In 1828, Friedrich Wöhler, later professor of chemistry at the University of Göttingen in Germany, heated the inorganic compound ammonia with cyanic acid to produce crystals of urea that were exactly the same as the organic compound urea isolated from animal urine.
Although vitalists argued that cyanic acid was organic because it was obtained from dried blood, the theory of vitalism began to crack. Over the next few decades it shattered completely as other chemists were able to produce organic compounds from totally inorganic sources. Though some scientists were reluctant to believe what seemed to be heresy, eventually the death of vitalism was commonly acknowledged. A new chemical definition of the word
organic
was needed.
organic
was needed.
Organic compounds are now defined as compounds that contain the element carbon. Organic chemistry, therefore, is the study of the compounds of carbon. This is not a perfect definition, however, as there are a number of carbon-containing compounds that chemists have never considered organic. The reason for this is mainly traditional. Carbonates, compounds with carbon and oxygen, were known to come from mineral sources and not necessarily from living things well before Wöhler's defining experiment. So marble (or calcium carbonate) and baking soda (sodium bicarbonate) have never been labeled organic. Similarly, the element carbon itself, either in the form of diamond or graphiteâboth originally mined from deposits in the ground although now also made syntheticallyâhas always been thought of as inorganic. Carbon dioxide, containing one carbon atom joined to two oxygen atoms, has been known for centuries but has never been classified as an organic compound. Thus the definition of
organic
is not completely consistent. But in general an organic compound is a compound that contains carbon, and an inorganic compound is one that consists of elements other than carbon.
organic
is not completely consistent. But in general an organic compound is a compound that contains carbon, and an inorganic compound is one that consists of elements other than carbon.
More than any other element, carbon has tremendous variability in the ways it forms bonds and also in the number of other elements to which it is able to bond. Thus there are many, many more compounds of carbon, both naturally occurring and man-made, than there are compounds of all the other elements combined. This may account for the fact that we will be dealing with many more organic than inorganic molecules in this book; or perhaps it is because both the authors are organic chemists.
CHEMICAL STRUCTURES: DO WE HAVE TO?In writing this book, our biggest problem was determining how much chemistry to include in its pages. Some people advised us to minimize the chemistry, to leave it out and just tell the stories. Especially, we've been told, do not draw any chemical structures. But it is the connection between chemical structures and what they do, between how and why a compound has the chemical properties it has, and how and why that affected certain events in history, that we find the most fascinating. While you can certainly read this book without looking at the structures, we think understanding the chemical structures makes the interwoven relationship between chemistry and history come alive.
Organic compounds are mainly composed of only a few types of atoms: carbon (with chemical symbol C), hydrogen (H), oxygen (O), and nitrogen (N). Other elements may be present as well; for example, bromine (Br), chlorine (Cl), fluorine (F), iodine (I), phosphorus (P), and sulfur (S) are also found in organic compounds. The structures in this book are generally drawn to illustrate differences or similarities between compounds; mostly all that is required is to look at the drawing. The variation will often be arrowed, circled, or indicated in some other way. For example, the only difference between the two structures shown below is in the position where OH is attached to a C; it's pointed out by an arrow in each case. For the first molecule the OH is on the second C from the left; for the second molecule the OH is attached to the first C from the left.
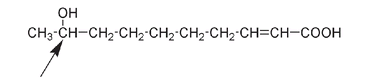
Molecule produced by honeybee queen
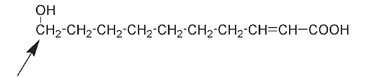
Molecule produced by honeybee worker
This is a very small difference, but is hugely important if you happen to be a honeybee. Queen honeybees produce the first molecule. Bees are able to recognize the difference between it and the second molecule, which is produced by honeybee workers. We can tell the difference between workers and queens by looking at the bees.
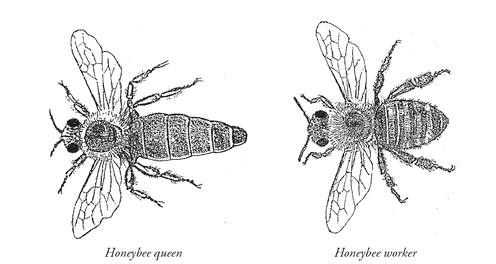
(Courtesy of Raymond and Sylvia Chamberlin)
Bees use chemical signaling to tell the difference. We could say they see through chemistry.
Chemists draw such structures to depict the way atoms are joined to each other through chemical bonds. Chemical symbols represent atoms, and bonds are drawn as straight lines. Sometimes there is more than one bond between the same two atoms; if there are two it is a double bond and shown as =. When three chemical links exist between the same two atoms, it is a triple bond and drawn as â¡.
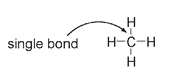
In one of the simplest organic molecules, methane (or marsh gas), carbon is surrounded by four single bonds, one to each of four hydrogen atoms. The chemical formula is given as CH
4
, and the structure is drawn as:
4
, and the structure is drawn as:
Other books
Hurt Me by Glenna Marie
An Acceptable Time by Madeleine L'Engle
Archangel Down: Archangel Project. Book One by C. Gockel
El cura de Tours by Honoré de Balzac
Roz Denny Fox by Precious Gifts
Emmanuelle by Emmanuelle Arsan
Raisonne Curse by Rinda Elliott
Blackstrap Hawco by Kenneth J. Harvey
The Quality of the Informant by Gerald Petievich
Legacy by James A. Michener