Penny le Couteur & Jay Burreson (3 page)
Read Penny le Couteur & Jay Burreson Online
Authors: Napoleon's Buttons: How 17 Molecules Changed History
Tags: #Philosophy & Social Aspects, #Science, #General, #World, #Chemistry, #Popular Works, #History

Methane
The simplest organic compound that has a double bond is ethene (also called ethylene) with a formula of C
2
H
4
and the structure:
2
H
4
and the structure:
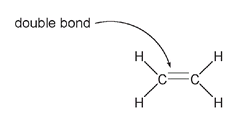
Ethylene
Here carbon still has four bondsâthe double bond counts as two. Despite being a simple compound, ethylene is very important. It is a plant hormone that is responsible for promoting the ripening of fruit. If apples, for example, aren't stored with appropriate ventilation, the ethylene gas they produce will build up and cause them to overripen. This is why you can hasten the ripening of a hard avocado or kiwi fruit by putting it in a bag with an already ripe apple. Ethylene produced by the mature apple increases the rate of ripening of the other fruit.
The organic compound methanol, also known as methyl alcohol or wood alcohol, has the formula CH
4
O. This molecule contains an oxygen atom and the structure is drawn as:
4
O. This molecule contains an oxygen atom and the structure is drawn as:
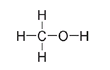
Methanol
Here the oxygen atom, O, has two single bonds, one connected to the carbon atom and the other to a hydrogen atom. As always,carbon has a total of four bonds.
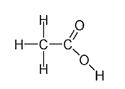
In compounds where there is a double bond between a carbon atom and an oxygen atom, as in acetic acid (the acid of vinegar), the formula, written as C
2
H
4
O
2
, does not directly indicate where the double bond is. This is the reason we draw chemical structuresâto show exactly which atom is attached to which other atom and where the double or triple bonds are.
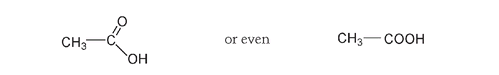 where not all the bonds are shown. They are, of course, still there, but these shortened forms are faster to draw and show just as clearly the relationships among atoms.
where not all the bonds are shown. They are, of course, still there, but these shortened forms are faster to draw and show just as clearly the relationships among atoms.
2
H
4
O
2
, does not directly indicate where the double bond is. This is the reason we draw chemical structuresâto show exactly which atom is attached to which other atom and where the double or triple bonds are.

Acetic acid
We can draw these structures in an abbreviated or more condensed form. Acetic acid could also be drawn as: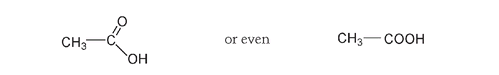
This system of drawing structures works well for smaller examples, but when the molecules get bigger, it becomes time consuming and difficult to follow. For example, if we return to the queen honeybee recognition molecule:
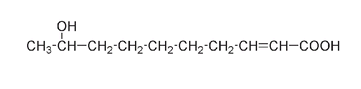 and compare it to a fully drawn-out version showing all the bonds, the structure would look like:
and compare it to a fully drawn-out version showing all the bonds, the structure would look like:
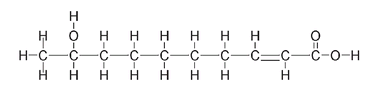
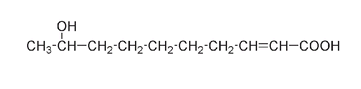

Fully drawn-out structure of the queen honeybee molecule
This full structure is cumbersome to draw and looks very cluttered. For this reason, we often draw compounds using a number of shortcuts, the most common of which is to leave out many of the H atoms. This does not mean that they are not there; we just do not show them. A carbon atom always has four bonds, so if it does not look as if C has four bonds, be assured it doesâthe ones that are not shown bond to hydrogen atoms.
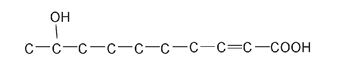
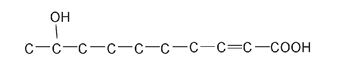
Queen honeybee recognition molecule
As well, the carbon atoms are often shown joined at an angle instead of in a straight line; this is more indicative of the true shape of the molecule. In this format the queen honeybee molecule looks like this:
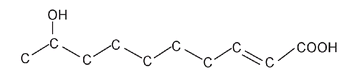

An even more simplified version leaves out most of the carbon atoms:
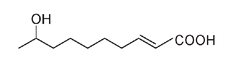
Here the end of a line and any intersection represent a carbon atom. All other atoms (except most of the carbons and hydrogens) are still shown. By simplifying in this manner, it is easier to see the difference between the queen molecule and the worker molecule.
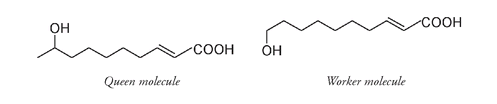
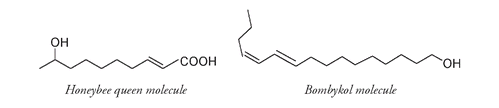
It is now also easier to compare these compounds to those emitted by other insects. For example,
bombykol,
the pheromone or sex attractant molecule produced by the male silkworm moth, has sixteen carbon atoms (as opposed to the ten atoms in the honeybee queen molecule, also a pheromone), has two double bonds instead of one, and lacks the COOH arrangement.
bombykol,
the pheromone or sex attractant molecule produced by the male silkworm moth, has sixteen carbon atoms (as opposed to the ten atoms in the honeybee queen molecule, also a pheromone), has two double bonds instead of one, and lacks the COOH arrangement.
Other books
Between Friends by Harper, Jenny
Death in Ecstasy by Ngaio Marsh
Mrs. Lincoln's Dressmaker by Jennifer Chiaverini
Carter (Remington Ranch Book 3) by SJ McCoy
Backlash by Lynda La Plante
Bride for Glenmore by Sarah Morgan
The Pearl Diver by Sujata Massey
When Truth Fails by Lucianna Gray
Inarticulate by Eden Summers
Scarlet Fever - Hill Country 2 by Hunter, Sable