Penny le Couteur & Jay Burreson (36 page)
Read Penny le Couteur & Jay Burreson Online
Authors: Napoleon's Buttons: How 17 Molecules Changed History
Tags: #Philosophy & Social Aspects, #Science, #General, #World, #Chemistry, #Popular Works, #History

Back in Pennsylvania he extracted a very similar sapogenin to the sarsasapogenin from the sarsaparilla plant. The only difference was an extra double bond (arrowed) found in diosgenin, the sapogenin from the wild yam.
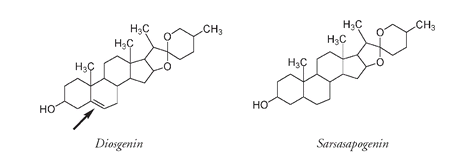
Diosgenin, from the Mexican yam, differs from the sarsaparilla sapogenin, sarsasapogenin, only by an extra double bond (arrowed).
The Marker degradation removed the unwanted side group, and further chemical reactions produced a generous amount of progesterone. Marker was convinced that the way to obtain sizable amounts of steroidal hormones at a reasonable cost would be to set up a laboratory in Mexico and use the abundant source in the Mexican yam.
But if this solution seemed practical and sensible to Marker, it did not appear that way to the major pharmaceutical companies that he tried to interest in his scheme. Tradition and authority once more blocked his way. Mexico had no history of carrying out such complicated chemical syntheses, the drug company authorities told him. Unable to get financial backing from established companies, Marker resolved to enter the hormone-production business himself. He resigned from Pennsylvania State College and eventually moved to Mexico City, where, in 1944 and in partnership with others, he established Syntex (for Synthesis and Mexico), the pharmaceutical company that was to become a world leader in steroidal products.
But Marker's relationship with Syntex was not to last. Arguments over payments, profits, and patents led to his departure. Another company he established, Botanica-Mex, was ultimately bought out by Eu-ropean drug companies. By this time Marker had discovered other species of
Dioscorea
that were even richer in the steroid-containing diosgenin molecule. The cost of synthetic progesterone steadily decreased. These yams, once an obscure root used only as a fish poison by local farmersâthe fish became dazed but were still edibleâare now grown as a commercial crop in Mexico.
Dioscorea
that were even richer in the steroid-containing diosgenin molecule. The cost of synthetic progesterone steadily decreased. These yams, once an obscure root used only as a fish poison by local farmersâthe fish became dazed but were still edibleâare now grown as a commercial crop in Mexico.

Russell Marker, whose development of the series of chemical steps known as the Marker degradation allowed chemists access to abundant plant steroid molecules.
(Photo courtesy of Pennsylvania State University)
(Photo courtesy of Pennsylvania State University)
Marker had always been reluctant to patent his procedures, feeling his discoveries should be available to everyone. By 1949 he was so disgusted and disappointed both with his fellow chemists and with the profit motive that he now saw as driving chemical research that he destroyed all his laboratory notes and experiment records in an attempt to remove himself totally from the field of chemistry. Despite these efforts the chemical reactions Marker pioneered are today acknowledged as the work that made possible the birth control pill.
SYNTHESIS OF OTHER STEROIDSIn 1949 a young Austrian immigrant to the United States joined the Syntex research facilities in Mexico City. Carl Djerassi had just finished his Ph.D. at the University of Wisconsin, where his thesis work involved the chemical conversion of testosterone to estradiol. Syntex wanted to find a way to convert the now relatively abundant progesterone from wild yams to the cortisone molecule. Cortisone is one of at least twenty-eight different hormones isolated from the adrenal cortex (the outer part of the adrenal glands adjacent to the kidneys). It is a potent anti-inflammatory agent that is especially effective in treating rheumatoid arthritis. Like other steroids, cortisone is present in minute quantities in animal tissues. Although it could be made in the laboratory, such methods were very expensive. Synthesis required thirty-two steps, and its starting material, desoxycholic acid, had to be isolated from ox bileâwhich was not at all abundant.
Using the Marker degradation, Djerassi showed how cortisone could be produced at a much lower cost from a plant source like diosgenin. One of the major stumbling blocks in making cortisone is attaching the double-bonded oxygen at carbon number 11 on the C ring, a position that is not substituted in the bile acids or sex hormones.
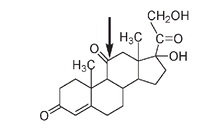
Cortisone. The C=O at C#11 is arrowed.
A novel method of attaching oxygen at this position was later discovered using the mold
Rhizopus nigricans.
The effect of this combination of fungi and chemist was to produce cortisone from progesterone in a total of only eight stepsâone microbiological and seven chemical.
Rhizopus nigricans.
The effect of this combination of fungi and chemist was to produce cortisone from progesterone in a total of only eight stepsâone microbiological and seven chemical.
After his success in making cortisone, Djerassi synthesized both estrone and estradiol from diosgenin, giving Syntex a preeminent position as a major world supplier of hormones and steroids. His next project was to make an artificial progestin, a compound that would have progesteronelike properties but could be taken orally. The aim was not to create a contraceptive pill. Progesterone, now available at a reasonable costâless than a dollar a gramâwas being used to treat women who had a history of miscarriage. It had to be injected and in fairly large doses. Djerassi's reading of the scientific literature led him to suspect that substituting, on the D ring, a group with a carbon-to-carbon triple bond (â¡) might allow the molecule to retain its effectiveness when swallowed. Another report had mentioned that removal of a CH
3
groupâthe carbon designated as number 19âseemed to increase potency in other progesteronelike molecules. The molecule Djerassi and his team produced and patented in November 1951 was eight times more powerful than progesterone and could be taken orally. It was named norethindroneâthe
nor
indicating a missing CH
3
group.
3
groupâthe carbon designated as number 19âseemed to increase potency in other progesteronelike molecules. The molecule Djerassi and his team produced and patented in November 1951 was eight times more powerful than progesterone and could be taken orally. It was named norethindroneâthe
nor
indicating a missing CH
3
group.
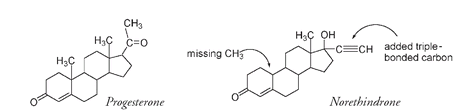
The structure of natural progesterone compared with the artificial progestin norethindrone
Critics of the birth control pill have pointed out that it was developed by men to be taken by women. Indeed, the chemists involved in the synthesis of the molecule that became the pill were men, but as Djerassi, who is now sometimes known as the “Father of the Pill,” was to say years later, “Not in our wildest dreams did we imagine that this substance would eventually become the active ingredient of nearly half the oral contraceptives used worldwide.” Norethindrone was designed as a hormone treatment to support pregnancy or to relieve menstrual irregularity, especially where severe blood loss was involved. Then in the early 1950s two women became the driving force responsible for changing the role of this molecule from a limited infertility treatment to an everyday factor in the lives of countless millions of women.
THE MOTHERS OF THE PILLMargaret Sanger, the founder of International Planned Parenthood, was jailed in 1917 for giving contraceptives to immigrant women at a Brooklyn clinic. Throughout her life she was an impassioned believer in a woman's right to control her own body and fertility. Katherine McCormick was one of the first women to receive a degree in biology from the Massachusetts Institute of Technology. She was also, after the death of her husband, immensely wealthy. She had known Margaret Sanger for over thirty years, had even helped her smuggle illegal contraceptive diaphragms into the United States, and had supplied financial help for the birth control cause. Both women were in their seventies when they journeyed to Shrewsbury, Massachusetts, for a meeting with Gregory Pincus, a specialist in female fertility and one of the founders of a small nonprofit organization called the Worcester Foundation for Experimental Biology. Sanger challenged Dr. Pincus to produce a safe, cheap, reliable “perfect contraceptive” that could be “swallowed like an aspirin.” McCormick supported her friend's venture with financial backing and over the next fifteen years contributed more than three million dollars to the cause.
Pincus and his colleagues at the Worcester Foundation first verified that progesterone did inhibit ovulation. Their work was done with rabbits; it was not until Pincus met another reproduction researcher, Dr. John Rock of Harvard University, that he realized similar results were available from human subjects. Rock was a gynecologist working to overcome fertility problems in his patients. His rationale for using progesterone to treat infertility assumed that blocking fertility by inhibiting ovulation for a few months would promote a “rebound effect” once the progesterone injections stopped.
In 1952 the state of Massachusetts had some of the most restrictive birth control laws in the United States. It was not illegal to use birth control, but exhibiting, selling, prescribing, and providing contraceptives and even information about contraception were all felonies. This law was not repealed until March 1972. Given these legal constraints, Rock was understandably cautious in explaining his progesterone-injection treatment to his patients. As the procedure was still experimental, informed consent was especially necessary. So the repression of ovulation was explained but was stressed as a temporary side effect to the real aim of increased fertility.
Neither Rock nor Pincus felt that injections of fairly large doses of progesterone would make a feasible long-term contraceptive. Pincus began contacting drug companies to find out if any of the artificial progesterones developed so far might be more potent in smaller doses and also effective orally. The answer came back: there were two synthetic progestins that fitted the requirements. The Chicago-based pharmaceutical company G. D. Searle had patented a molecule very similar to that synthesized by Djerassi at Syntex. Their norethynodrel differed from norethindrone only in the position of a double bond. The effective molecule is assumed to be norethindrone; stomach acids supposedly flipped the position of the double bond of norethynodrel to that of its structural isomerâsame formula, different arrangementânorethindrone.
Other books
Romance: What The Billionaire Wants by Ward, Elizabeth
A Death in Belmont by Sebastian Junger
Faerie Wars 02 - The Purple Emperor by Brennan, Herbie
The Serpent's Curse by Tony Abbott
Deeply, Desperately by Heather Webber
The Irish Cottage Murder by Dicey Deere
Family Honor by Jamie Hill
A Knight's Persuasion by Catherine Kean
Desert of the Damned by Nelson Nye
How to Kill Yourself in a Small Town by eden Hudson