Examination Medicine: A Guide to Physician Training (29 page)
Read Examination Medicine: A Guide to Physician Training Online
Authors: Nicholas J. Talley,Simon O’connor
Tags: #Medical, #Internal Medicine, #Diagnosis

FIGURE 6.12
(a) Cystic fibrosis PA and (b) lateral films in a young patient. Note increased lung markings in the right middle lobe with ‘tram tracking’ – increased bronchial wall thickness (arrows). Figures reproduced courtesy of The Canberra Hospital.
5.
Chest CT is not routine but may help define focal areas of bronchiectasis that are amenable to resection.
6.
Spirometry readings may fluctuate because of airway inflammation. An FEV
1
that is persistently less than 40% indicates a poor prognosis.
Management
Try to form an idea of the patient’s ability to cope with the illness, as so much of the management depends on this.
1.
Intensive and repetitive physiotherapy is the mainstay of treatment. Inactivated influenza vaccine and the pneumococcal vaccine should be routine.
2.
Intravenous antibiotics may be indicated for acute exacerbations of pulmonary disease if these are severe or fail to respond to oral antibiotic treatment. Intravenous options include tobramycin and ceftazimide for
Pseudomonas
. Inhaled tobramycin seems useful and bronchodilators can help airway clearance. Nebulised anti-pseudomonas antibiotics improve lung function and prognosis.
3.
Malabsorption may require aggressive treatment with pancreatic enzyme supplements, including lipase, and frequent small meals as well as vitamin supplements.
4.
Double lung transplantation is now an accepted, although still uncommon, treatment in patients with advanced disease. Cystic fibrosis does not recur in the transplanted lung. The 5-year survival rate is only about 65%.
5.
Human recombinant DNAase seems effective in degrading the concentration of DNA in sputum, reducing sputum viscosity and improving the patient’s ability to clear pulmonary secretions. Gene therapy may be the treatment of choice in the future (e.g. ivacaftor for the G551D mutation).
Tuberculosis
The increased incidence of pulmonary tuberculosis (TB) over the past 10 years or so and its association with HIV infection have made it a possible long case. Protection from
Mycobacterium tuberculosis
is via cell-mediated immunity involving CD4
+
T-helper lymphocytes. The defects of these cells in number and effectiveness, which is characteristic of HIV infection, explain this susceptibility. It may be a difficult diagnostic or management problem, or both. It also has important social and public health implications.
The history
The patient is likely to know that the diagnosis has been made or is suspected.
1.
Find out whether the patient is a recent immigrant and, if so, from where.
2.
Ask whether there has been a previous diagnosis of any other serious medical problem that might interfere with cell-mediated immunity, such as malnutrition, alcoholism or HIV infection.
3.
Establish how the diagnosis was made and how long ago.
4.
Ask about some of the important symptoms – weight loss, sweats and fever, cough with purulent and blood-stained sputum, and pleuritic chest pain from pleural lesions or uncontrolled coughing.
5.
Ask what investigations have been performed or are planned. TB may have been suspected after a chest X-ray was performed because of respiratory symptoms or as a routine screening test. The patient may remember having to give early morning sputum specimens on a number of occasions or having had a bronchocospy with washings, if sputum was not being produced. Bronchoscopy may have been performed to exclude other causes of an abnormal chest X-ray, such as carcinoma of the lung.
A tuberculin (Mantoux) skin test may have been performed as a screening test or to support the diagnosis where cultures have been negative. A positive tuberculin test is still significant, even if the patient has received a bacille Calmette-Guérin (BCG) vaccination in the past. Remember, if the patient is in a high-risk group (HIV positive, immunosuppressed, in close contact with a clear-cut case of TB or has evidence of prior TB on chest X-ray), the Mantoux test is considered positive with just 5 mm of induration (vs 15 mm in low-risk patients).
6.
Find out what treatment regimen the patient is receiving. Considerable detail must be sought about the drugs themselves – doses (if known), how long treatment will continue, how the drugs are administered (supervised or unsupervised) and what side-effects have occurred (
Table 6.18
). Remember that rifampicin and rifabutin colour body fluids, including urine, orange.
Table 6.18
Common antituberculous drugs and their side-effects
| DRUG | MAIN SIDE-EFFECTS | USUAL DOSE/DAY |
| Rifampicin | Hepatitis, flu-like illness | 600 mg |
| Isoniazid | Hepatitis, fever, peripheral neuropathy | 300 mg (reduced to 2 or 3 doses a week in renal failure) |
| Streptomycin | Ototoxicity, renal impairment | 1 g (not safe in pregnancy) |
| Ethambutol | Optic neuritis | 15 mg/kg |
| Pyrazinamide | Hepatitis | 1.5–2 g |
| PAS | Hepatitis, diarrhoea, hypersensitivity | 12 g |
PAS = para-aminosalicylic acid.
7.
Ask whether there has been a trigger for reactivation of previous infection (diabetes mellitus, old age, HIV infection).
8.
Find out what effect this serious and chronic disease has had on the patient’s life. Has the patient been able to work or go to school? Do friends and workmates know the diagnosis? Does the patient’s occupation involve a public health risk?
9.
Establish how the problem has been handled from a public health aspect. What screening has been done on the patient’s family and friends? Are any of the family also being treated?
The examination
Early in the course of the illness there may be no specific signs. Of those patients without HIV infection who have TB, 80% have only pulmonary disease. However, the majority of patients with HIV and TB have extrapulmonary and pulmonary disease.
1.
Note the patient’s general appearance. Look for wasting and cachexia that may be associated with the risk factors for TB (HIV, alcoholism) or may be a result of the disease (which is also known as consumption for this reason).
2.
Examine the lungs for signs of the aggressive primary infection that can occur in immunocompromised patients. These include a pleural effusion or tuberculous empyema and lobar collapse (as a result of lymphadenopathy and bronchial obstruction).
3.
Post-primary disease is more common in adults. There is almost always a loose cough and often haemoptysis. There may be no abnormal findings, but you should look
especially for upper lobe signs, including coarse crackles and wheezes owing to partial bronchial obstruction caused by lymphadenopathy, and the rare amphoric breath sounds that occur over a cavity.
4.
Extrapulmonary disease most commonly involves the lymph nodes (especially in HIV patients). Examine all the palpable lymph node groups. The most often affected are the cervical and supraclavicular. There is usually painless swelling.
5.
Involvement of the genitourinary tract can be associated with haematuria and tenderness over the flanks.
6.
The bones are involved in a small number of cases. The lumbar spine (Pott’s disease), hips and knees are most affected. Collapse of the vertebral bodies may cause kyphosis and even paraplegia.
7.
Tuberculous pericarditis can cause symptoms and signs of pericarditis and occasionally tamponade.
8.
Abdominal TB can cause various gastrointestinal signs. There may be a palpable abdominal mass. Severe abdominal tenderness suggests tuberculous peritonitis.
Investigations
1.
The diagnosis of active disease depends on microscopy of a specimen that shows mycobacteria (e.g. send three morning sputum specimens on separate days, followed by confirmation by culture of the organism). Traditionally, specimens of sputum or gastric or bronchial washings (often useful for children), or a lymph node biopsy, are stained with the Ziehl-Neelsen stain. Modern laboratories are more likely to use auramine-rhodamine stains and fluorescence microscopy. Only about 60% of patients with eventually proven TB have positive microscopy. Culture of the organism takes about 6 weeks, but is needed for the definitive diagnosis. These organisms should then be tested for sensitivity to the main antituberculous drugs. This takes up to 8 more weeks. If multidrug resistance (MDRTB) is suspected, the
rpo
gene can be tested: it is responsible for 95% of rifampicin resistance. Polymerase chain reaction (PCR) testing can now provide rapid identification of
M. tuberculosis
, but is not available everywhere and does not distinguish between viable and non-viable organisms.
2.
A chest X-ray showing the typical pattern of infiltrates and cavities in the upper lobe strongly suggests the diagnosis, but many other patterns are consistent with TB (
Figs 6.13
,
6.14
and
6.15
).
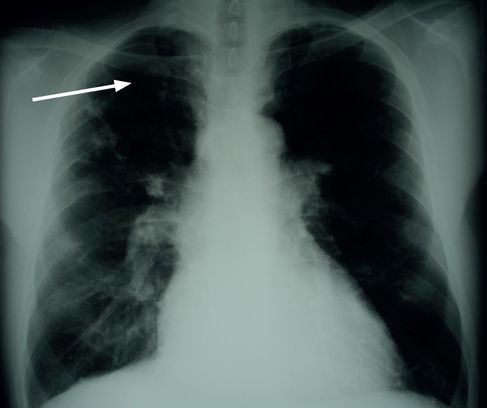
FIGURE 6.13
Right upper lobe scarring; old TB infection (arrow). Figure reproduced courtesy of The Canberra Hospital.
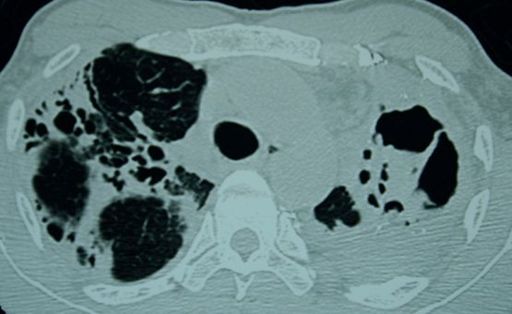
FIGURE 6.14
CT scan of the thorax in a patient with TB; note the destructive changes and cavitation. Figure reproduced courtesy of The Canberra Hospital.
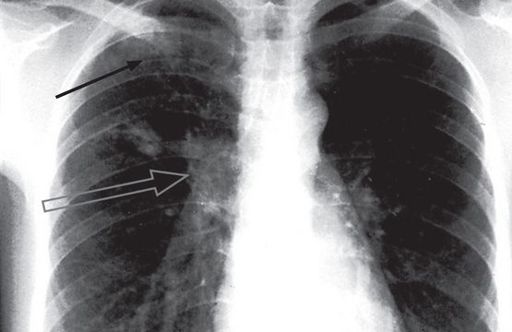
FIGURE 6.15
Pulmonary tuberculosis. Two small rounded areas of shadowing are seen in the right upper zone (black arrow). The right hilum is enlarged by the enlarged draining lymph nodes (open arrow). This combination of focal shadowing and enlarged lymph nodes is the primary (Ghon) complex of tuberculosis. With healing, calcification may occur in the parenchymal and nodal lesions. In contrast, in tuberculosis reactivation or re-infection, cavitation may occur and there is no lymphadenopathy. The Canberra Hospital X-Ray Library, reproduced with permission.
3.
Tuberculin (Mantoux) skin testing is useful for screening for latent TB. The test may be negative in severe disease (e.g. in up to 50% of cases of miliary TB) or if exposure is only recent (repeat in 3 months). IFNλ release assays use a specific tuberculosis antigen to stimulate release of IFNλ from T cells in vitro. These tests are more specific than tuberculin testing and may be useful where rates of TB are low. They are not affected by previous BCG vaccination. A positive screening test should be followed by a chest X-ray to look for active disease.