Penny le Couteur & Jay Burreson (12 page)
Read Penny le Couteur & Jay Burreson Online
Authors: Napoleon's Buttons: How 17 Molecules Changed History
Tags: #Philosophy & Social Aspects, #Science, #General, #World, #Chemistry, #Popular Works, #History

SWEET NOTHING
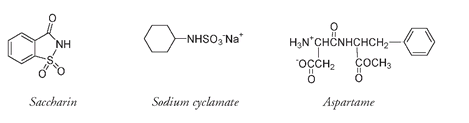
There are numerous other nonsugars that taste sweet, and some of these compounds are the basis for the billion-dollar artificial sweetener industry. As well as having a chemical structure that in some way mimics the geometry of sugars, allowing it to fit and bind to the sweetness receptor, an artificial sweetener needs to be water soluble and nontoxic and, often, not metabolized in the human body. These substances are usually hundreds of times sweeter than sugar.
The first of the modern artificial sweeteners to be developed was saccharin. Saccharin is a fine powder. Those who work with it sometimes detect a sweet taste if they accidentally touch their fingers to their mouth. It is so sweet that only a very small amount triggers the sweetness response. This is evidently what happened in 1879, when a chemistry student at Johns Hopkins University in Baltimore noticed an unusual sweetness in the bread he was eating. He returned to his laboratory bench to systematically taste the compounds that he had been using in that day's experimentsâa risky but common practice with new molecules in those daysâand discovered that saccharin was intensely sweet.
Saccharin has no calorific value, and it did not take long (1885) for this combination of sweetness and no calories to be commercially exploited. Originally intended as a replacement for sugar in the diet of diabetic patients, it quickly became an accepted sugar substitute for the general population. Concern about possible toxicity and the problem of a metallic aftertaste led to the development of other artificial sweeteners, such as cyclamate and aspartame. As you can see, the structures of these are all quite different and are very different from sugars, yet they all have the appropriate atoms, along with the specific atomic position, geometry, and flexibility that is necessary for sweetness.

No artificial sweetener is completely free of problems. Some decompose on heating and so can be used only in soft drinks or cold foods; some are not particularly soluble; and others have a detectable side taste along with their sweetness. Aspartame, although synthetic, is composed of two naturally occurring amino acids. It is metabolized by the body, but as it is over two hundred times sweeter than glucose, a lot less is needed to produce a satisfactory level of sweetness. Those with the inherited condition known as PKU (phenylketonuria), an inability to metabolize the amino acid phenylalanine, one of the breakdown products of aspartame, are told to avoid this particular artificial sweetener.
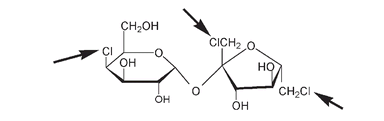
A new sweetener that was approved by the U.S. Food and Drug Administration in 1998 approaches the problem of creating artificial sweetness in a different way. Sucralose has a very similar structure to that of sucrose except for two factors. The glucose unit, on the left-hand side in the diagram, is replaced by galactose, the same unit as in lactose. Three chlorine atoms (Cl) replace three of the OH groups: one on the galactose unit and the other two on the right-hand fructose unit, as indicated. The three chlorine atoms do not affect the sweetness of this sugar, but they do stop the body from metabolizing it. Hence sucralose is a noncalorific sugar.

Sucralose structure, showing the three Cl atoms (arrows) replacing three OHs
Natural nonsugar sweeteners are now being sought from plant sources containing “high-potency sweeteners”âcompounds that can be as much as a thousand times sweeter than sucrose. For centuries indigenous people have known about plants that have a sweet taste; the South American herb
Stevia rebaudiana;
roots of the licorice plant
Glycyrrhiza glabra; Lippia dulcis,
a Mexican member of the verbena family; and rhizomes from
Selliguea feei,
a fern from West Java, are examples. Sweet compounds from natural sources have shown potential for commercial application, but problems with small concentrations, toxicity, low water solubility, unacceptable aftertaste, stability, and variable quality still need to be overcome.
Stevia rebaudiana;
roots of the licorice plant
Glycyrrhiza glabra; Lippia dulcis,
a Mexican member of the verbena family; and rhizomes from
Selliguea feei,
a fern from West Java, are examples. Sweet compounds from natural sources have shown potential for commercial application, but problems with small concentrations, toxicity, low water solubility, unacceptable aftertaste, stability, and variable quality still need to be overcome.
While saccharin has been used for more than a hundred years, it was not the first substance to be used as an artificial sweetener. That distinction probably belongs to lead acetate, Pb(C
2
H
3
O
2
)
2
, which was used to sweeten wine in the days of the Roman Empire. Lead acetate, known as sugar of lead, would sweeten a vintage without causing further fermentation, which would have occurred with the addition of sweeteners like honey. Lead salts are known to be sweet, and many are insoluble, but all are poisonous. Lead acetate is very soluble, and its toxicity was obviously not known to the Romans. This should give us pause to think, if we long for the good old days when food and drink were uncontaminated with additives.
2
H
3
O
2
)
2
, which was used to sweeten wine in the days of the Roman Empire. Lead acetate, known as sugar of lead, would sweeten a vintage without causing further fermentation, which would have occurred with the addition of sweeteners like honey. Lead salts are known to be sweet, and many are insoluble, but all are poisonous. Lead acetate is very soluble, and its toxicity was obviously not known to the Romans. This should give us pause to think, if we long for the good old days when food and drink were uncontaminated with additives.
The Romans also stored wine and other beverages in lead containers and supplied their houses with water through lead pipes. Lead poisoning is cumulative. It affects the nervous system and the reproductive system as well as other organs. Initial symptoms of lead poisoning are vague but include restless sleep, loss of appetite, irritation, headaches, stomach-aches, and anemia. Brain damage, leading to gross mental instability and paralysis, develops. Some historians have attributed the fall of the Roman Empire to lead poisoning, as many Roman leaders, including the Emperor Nero, are reported to have exhibited these symptoms. Only the wealthy, aristocratic, ruling Roman class had water piped to their houses and used lead vessels for storing wine. Ordinary people would have fetched their water and stored their wine in other containers. If lead poisoning did indeed contribute to the fall of the Roman Empire, it would be yet another example of a chemical that changed the course of history.
Sugarâthe desire for its sweetnessâshaped human history. It was profit from the huge sugar market developing in Europe that motivated the shipping of African slaves to the New World. Without sugar there would have been a much-reduced slave trade; without slaves there would have been a much-reduced sugar trade. Sugar started the massive buildup of slavery, and sugar revenues sustained it. The wealth of West African statesâtheir peopleâwas transferred to the New World to build wealth for others.
Even after slavery was abolished, the desire for sugar still affected human movement around the globe. At the end of the nineteenth century large numbers of indentured laborers from India went to the Fijian Islands to work in the sugarcane fields. As a result, the racial composition of this Pacific island group changed so completely that the native Melanesians were no longer a majority. After three coups in recent years Fiji is still a country of political and ethnic unrest. The racial makeup of the population of other tropical lands also owes much to sugar. Many of the ancestors of present-day Hawaii's largest ethnic group emigrated from Japan to work in the Hawaiian sugarcane fields.
Sugar continues to shape human society. It is an important trade commodity; vagaries in weather and pest infestations affect the economies of sugar-growing countries and stock markets around the world. An increase in sugar price has a ripple effect throughout the food industry. Sugar has been used as a political tool; for decades purchase of Cuban sugar by the USSR supported the economy of Fidel Castro's Cuba.
We have sugar in much of what we drink and much of what we eat. Our children prefer sugary treats. We tend to offer sweet foods when we entertain; offering hospitality to guests no longer means breaking a simple loaf of bread. Sugar-laden treats and candies are associated with major holidays and celebrations in cultures around the world. Levels of consumption of the glucose molecule and its isomers, many times higher than in previous generations, are reflected in health problems such as obesity, diabetes, and dental caries. In our everyday lives we continue to be shaped by sugar.
4. CELLULOSE
S
UGAR PRODUCTION promoted the buildup of the slave trade to the Americas, but sugar was not alone in sustaining it for over three centuries. The cultivation of other crops for the European market also depended on slavery. One of these crops was cotton. Raw cotton shipped to England could be made into the cheap manufactured goods that were sent to Africa in exchange for the slaves shipped to plantations in the New World, especially to the southern United States. Profit from sugar was the first fuel for this trade triangle, and it supplied the initial capital for the growing British industrialization. But it was cotton and the cotton trade that launched rapid economic expansion in Britain in the late eighteenth century and early nineteenth century.
COTTON AND THE INDUSTRIAL REVOLUTIONUGAR PRODUCTION promoted the buildup of the slave trade to the Americas, but sugar was not alone in sustaining it for over three centuries. The cultivation of other crops for the European market also depended on slavery. One of these crops was cotton. Raw cotton shipped to England could be made into the cheap manufactured goods that were sent to Africa in exchange for the slaves shipped to plantations in the New World, especially to the southern United States. Profit from sugar was the first fuel for this trade triangle, and it supplied the initial capital for the growing British industrialization. But it was cotton and the cotton trade that launched rapid economic expansion in Britain in the late eighteenth century and early nineteenth century.
The fruit of the cotton plant develops as a globular pod known as a boll, which contains oily seeds within a mass of cotton fibers. There is evidence that cotton plants, members of the
Gossypium
genus, were cultivated in India and Pakistan and also in Mexico and Peru some five thousand years ago, but the plant was unknown in Europe until around 300 B.C. when soldiers from the army of Alexander the Great returned from India with cotton robes. Arab traders brought cotton plants to Spain during the Middle Ages. The cotton plant is frost tender and needs moist but well-drained soil and long hot summers, not the conditions found in the temperate regions of Europe. Cotton had to be imported to Britain and other northern countries.
Gossypium
genus, were cultivated in India and Pakistan and also in Mexico and Peru some five thousand years ago, but the plant was unknown in Europe until around 300 B.C. when soldiers from the army of Alexander the Great returned from India with cotton robes. Arab traders brought cotton plants to Spain during the Middle Ages. The cotton plant is frost tender and needs moist but well-drained soil and long hot summers, not the conditions found in the temperate regions of Europe. Cotton had to be imported to Britain and other northern countries.
Lancashire, in England, became the center of the great industrial complex that grew up around cotton manufacture. The damp climate of the region helped cotton fibers stick together, which was perfect for the manufacture of cotton, as it meant less likelihood of threads breaking during the spinning and weaving processes. Cotton mills in drier climates suffered higher production costs due to this factor. As well, Lancashire had land available for building mills, land for housing the thousands who were needed to work in the cotton industry, abundant soft water for the bleaching, dyeing, and printing of cotton, and a plentiful supply of coal, a factor that became very important as steam power arrived.
In 1760, England imported 2.5 million pounds of raw cotton. Less than eighty years later the country's cotton mills were processing more than 140 times this amount. This increase had an enormous effect on industrialization. Demand for cheap cotton yarns led to mechanical innovation, and eventually all stages of cotton processing became mechanized. The eighteenth century saw the development of the mechanical cotton gin for separating the cotton fiber from the seeds, carding machines to prepare the raw fiber, the spinning jenny and spinning throstles for drawing out the fiber and twisting it into a thread, and various versions of mechanical shuttles for weaving. Soon these machines, initially powered by humans, were run by animals or by water wheels. The invention of the steam engine by James Watt led to the gradual introduction of steam as the main power source.
The social consequences of the cotton trade were enormous. Large areas of the English Midlands were transformed from a farming district with numerous small trading centers into a region of almost three hundred factory towns and villages. Working and living conditions were terrible. Very long hours were required of workers, under a factory system of strict rules and harsh discipline. While not quite the slavery that existed on the cotton plantations on the other side of the Atlantic, the cotton trade brought servitude, squalor, and misery to the many thousands who worked in the dusty, noisy, and dangerous cotton factories. Wages were often paid in overpriced goodsâworkers had no say in this practice. Housing conditions were deplorable; in areas around the factories buildings were crowded together along narrow, dark, and poorly drained lanes. Factory workers and their families were crammed into these cold, damp, and dirty accommodations, often two or three families to a house with another family in the cellar. Less than half of children born under these conditions survived to their fifth birthday. Some authorities were concerned, not because of the appallingly high infant mortality rate but because these children died “before they can be engaged in factory labor, or in any other labor whatsoever.” When children did reach an age to work in the cotton mills, where their small size allowed them to crawl underneath machines and their nimble fingers to repair breaks in the threads, they were often beaten to keep them awake for the twelve to fourteen hours of the working day.
Other books
Fallen Crest Alternative Version by Tijan
Dangerous (The Complete Erotic Romance Novel) by Ella Ardent
Futanari Legends: The Frozen Queen (Book 1: Brenna) by Angel Black
The Sentimental Agents in the Volyen Empire by Doris Lessing
Romano and Albright 01 - Catch Me If You Can (MM) by L.B. Gregg
The Garden of Stars by Zoe Chamberlain
El hijo del lobo by Jack London
On the Rocks by Erin Duffy
The Letting by Cathrine Goldstein
The Sweetest Revenge by Lucy Felthouse