Penny le Couteur & Jay Burreson (13 page)
Read Penny le Couteur & Jay Burreson Online
Authors: Napoleon's Buttons: How 17 Molecules Changed History
Tags: #Philosophy & Social Aspects, #Science, #General, #World, #Chemistry, #Popular Works, #History

Indignation over ill treatment of children and other abuses generated a widespread humanitarian movement pushing for laws governing work hours, child labor, and factory safety and health, from which much of our present-day industrial legislation evolved. The conditions encouraged many factory workers to take an active role in the trade union movement and numerous other movements for social, political, and educational reforms. Change did not come easily, however. Factory owners and their shareholders wielded enormous political power and were reluctant to accept any decrease in the huge profits from the cotton trade that might result from the cost of improving working conditions.
A pall of dark smoke from the hundreds of cotton mills was a permanent fixture over the city of Manchester, which grew and flourished along with the cotton trade. Cotton profits were used to further industrialize the region. Canals and railways were built to transport raw materials and coal to the factories and finished products to the nearby port of Liverpool. Demand grew for engineers, mechanics, builders, chemists, and artisansâthose with the technical skills needed by a vast manufacturing enterprise with products and services as diverse as dyestuffs, bleaches, iron foundries, metalworks, glassmaking, shipbuilding, and railway manufacturing.
Despite legislation enacted in England in 1807 abolishing the slave trade, industrialists did not hesitate to import slave-grown cotton from the American South. Raw cotton, from other cotton-producing countries such as Egypt and India as well as the United States, was Britain's largest import during the years between 1825 and 1873, but the processing of cotton declined when raw cotton supplies were cut off during World War I. The industry in Britain never recovered to its former levels because cotton-growing countries, installing more modern machinery and able to use less expensive local labor, became important producersâand significant consumersâof cotton fabric.
The sugar trade had provided the original capital for the Industrial Revolution, but much of the prosperity of nineteenth-century Britain was based on the demand for cotton. Cotton fabric was cheap and attractive, ideal for clothing and household furnishings. It could mix with other fibers without problems and was easy to wash and sew. Cotton quickly replaced the more expensive linen as the plant fiber of choice for a vast number of ordinary people. The huge increase in demand for raw cotton in Europe, especially in England, led to a great expansion of slavery in America. Cotton cultivation was very labor intensive. Agricultural mechanization, pesticides, and herbicides were much later inventions, so cotton plantations relied on the human labor supplied by slaves. In 1840 the slave population of the United States was estimated at 1.5 million. Just twenty years later, when raw cotton exports accounted for two-thirds of the total value of U.S. exports, there were four million slaves.
CELLULOSE, A STRUCTURAL POLYSACCHARIDELike other plant fibers, cotton consists of over 90 percent cellulose, which is a polymer of glucose and a major component of plant cell walls. The term
polymer
is often associated with synthetic fibers and plastics, but there are also many naturally occurring polymers. The word comes from two Greek words,
poly
meaning “many” and
meros
meaning “parts”âor unitsâso a polymer consists of many units. Polymers of glucose, also known as polysaccharides, can be classified on the basis of their function in a cell. Structural polysaccharides, like cellulose, provide a means of support for the organism; storage polysaccharides supply a way of storing glucose until it is needed. The units of structural polysaccharides are β-glucose units; those of storage polysaccharides are α-glucose. As discussed in Chapter 3, β refers to the OH group on carbon number 1 above the glucose ring. The structure of α-glucose has the OH at carbon number 1 below the ring.
polymer
is often associated with synthetic fibers and plastics, but there are also many naturally occurring polymers. The word comes from two Greek words,
poly
meaning “many” and
meros
meaning “parts”âor unitsâso a polymer consists of many units. Polymers of glucose, also known as polysaccharides, can be classified on the basis of their function in a cell. Structural polysaccharides, like cellulose, provide a means of support for the organism; storage polysaccharides supply a way of storing glucose until it is needed. The units of structural polysaccharides are β-glucose units; those of storage polysaccharides are α-glucose. As discussed in Chapter 3, β refers to the OH group on carbon number 1 above the glucose ring. The structure of α-glucose has the OH at carbon number 1 below the ring.
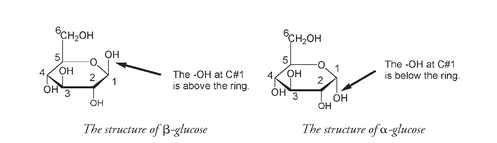
The difference between α- and β-glucose may seem small, but it is responsible for enormous differences in function and role among the various polysaccharides derived from each version of glucose: above the ring, structural; and below the ring, storage. That a very small change in the structure of a molecule can have profound consequences for properties of the compound is something that occurs again and again in chemistry. The α and β polymers of glucose demonstrate this observation extremely well.
In both structural and storage polysaccharides, the glucose units are joined to each other through carbon number 1 on a glucose molecule and carbon number 4 on the adjacent glucose molecule. This joining occurs by the removal of a molecule of water formed from an H of one of the glucose molecules and an OH from the other glucose molecule. The process is known as condensationâthus these polymers are known as condensation polymers.
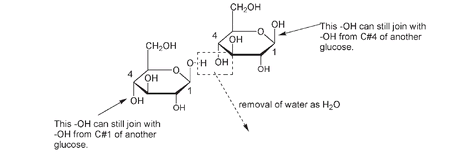
Condensation (loss of a molecule of water) between two
β
-glucose molecules. Each molecule can repeat this process again at its opposite end.
β
-glucose molecules. Each molecule can repeat this process again at its opposite end.
Each end of the molecule is able to join to another by condensation, forming long continuous chains of glucose units with the remaining OH groups distributed around the outside of the chain.
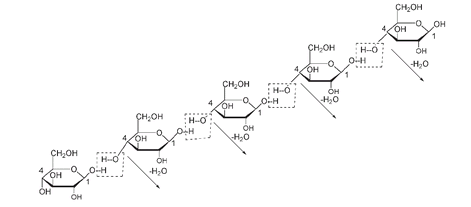
Elimination of a molecule of H
2
O between C#1 of one
β
-glucose and C#4 of the next forms a long polymer chain of cellulose. The diagram shows five
β
-glucose units.
2
O between C#1 of one
β
-glucose and C#4 of the next forms a long polymer chain of cellulose. The diagram shows five
β
-glucose units.
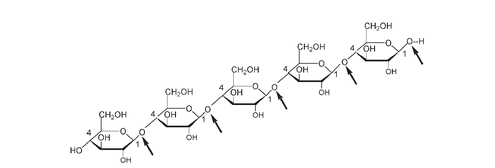
Structure of part of a long cellulose chain. The O attached at each C#1, as indicated with an arrow, is
β
, i.e., is above the ring to its left.
β
, i.e., is above the ring to its left.
Many of the traits that make cotton such a desirable fabric arise from the unique structure of cellulose. Long cellulose chains pack tightly together, forming the rigid, insoluble fiber of which plant cell walls are constructed. X-ray analysis and electron microscopy, techniques used to determine physical structures of substances, show that the cellulose chains lie side by side in bundles. The shape a β linkage confers on the structure allows the cellulose chains to pack closely enough to form these bundles, which then twist together to form fibers visible to the naked eye. On the outside of the bundles are the OH groups that have not taken part in the formation of the long cellulose chain, and these OH groups can attract water molecules. Thus cellulose can take up water, accounting for the high absorbency of cotton and other cellulose-based products. The statement that “cotton breathes” has nothing to do with the passage of air but everything to do with the absorbency of water by cotton. In hot weather perspiration from the body is absorbed by cotton garments as it evaporates, cooling us down. Clothes made from nylon or polyester don't absorb moisture, so perspiration is not “wicked” away from the body, leading to an uncomfortable humid state.
Another structural polysaccharide is
chitin,
a variation of cellulose found in the shells of crustaceans such as crabs, shrimps, and lobsters. Chitin, like cellulose, is a β-polysaccharide. It differs from cellulose only at the carbon number 2 position on each β-glucose unit, where the OH is replaced by an amide (NHCOCH
3
) group. So each unit of this structural polymer is a glucose molecule where NHCOCH
3
replaces OH at carbon number 2. The name of this molecule is N-acetyl glucosamine. This may not seem terribly interesting, but if you suffer from arthritis or other joint ailments, you may already know the name. N-acetyl glucosamine and its closely related derivative glucosamine, both manufactured from crustacean shell, have provided relief for many arthritis victims. They are thought to stimulate the replacement of or to supplement cartilaginous material in the joints.
chitin,
a variation of cellulose found in the shells of crustaceans such as crabs, shrimps, and lobsters. Chitin, like cellulose, is a β-polysaccharide. It differs from cellulose only at the carbon number 2 position on each β-glucose unit, where the OH is replaced by an amide (NHCOCH
3
) group. So each unit of this structural polymer is a glucose molecule where NHCOCH
3
replaces OH at carbon number 2. The name of this molecule is N-acetyl glucosamine. This may not seem terribly interesting, but if you suffer from arthritis or other joint ailments, you may already know the name. N-acetyl glucosamine and its closely related derivative glucosamine, both manufactured from crustacean shell, have provided relief for many arthritis victims. They are thought to stimulate the replacement of or to supplement cartilaginous material in the joints.

Fields of celluloseâa cotton field.
(Photo by Peter Le Couteur)
(Photo by Peter Le Couteur)
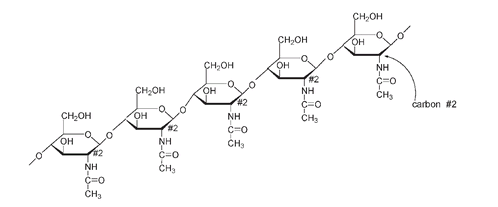
Part of the structure of the polymer chitin found in crustacean shells. At C#2 the OH of cellulose has been substituted by NHCOCH
3
.
3
.
Humans and all other mammals lack the digestive enzymes needed to break down β linkages in these structural polysaccharides, and so we cannot use them as a food source, despite the billions and billions of glucose units available as cellulose in the plant kingdom. But there are bacteria and protozoa that do produce the enzymes necessary to split the β linkage and are thus able to break down cellulose into its component glucose molecules. The digestive systems of some animals include temporary storage areas where these microorganisms live, enabling their hosts to obtain nourishment. For example, horses have a cecumâa large pouch where the small and large intestines connectâfor this purpose. Ruminants, a group that includes cattle and sheep, have a four-chambered stomach, one part of which contains the symbiotic bacteria. Such animals also periodically regurgitate and rechew their cud, another digestive system adaptation designed to improve access to the β linkage enzyme.
Other books
Saint Intervenes by Leslie Charteris
The Mark-2 Wife by William Trevor
The Pink Suit: A Novel by Nicole Kelby
Dark Nights by Christine Feehan
Welcome to Temptation by Jennifer Crusie
Fly by Night by Ward Larsen
Nice Day to Die by Cameron Jace
Sarny by Gary Paulsen
Eternity (Circle of Light) by April Margeson
Abducted by Adera Orfanelli