Penny le Couteur & Jay Burreson (11 page)
Read Penny le Couteur & Jay Burreson Online
Authors: Napoleon's Buttons: How 17 Molecules Changed History
Tags: #Philosophy & Social Aspects, #Science, #General, #World, #Chemistry, #Popular Works, #History

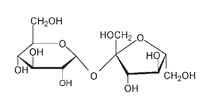
Structure of the sucrose molecule
Fructose is largely found in fruit but also in honey, which is about 38 percent fructose and 31 percent glucose, with another 10 percent of other sugars including sucrose. The remainder is mainly water. Fructose is sweeter than sucrose or glucose, so because of its fructose component, honey is sweeter than sugar. Maple syrup is approximately 62 percent sucrose with only 1 percent of each of fructose and glucose.
Lactose, also called milk sugar, is a disaccharide formed from one unit of glucose and one unit of another monosaccharide, galactose. Galactose is an isomer of glucose; the only difference is that in galactose the OH group at carbon number 4 is above the ring and not below the ring as it is in glucose.
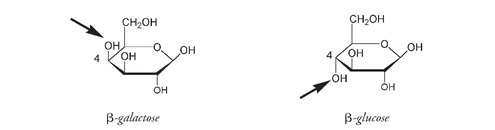
β
-galactose with arrow showing C#4 OH above the ring compared to
β
-glucose where the C#4 OH is below the ring. These two molecules combine to form lactose.
-galactose with arrow showing C#4 OH above the ring compared to
β
-glucose where the C#4 OH is below the ring. These two molecules combine to form lactose.
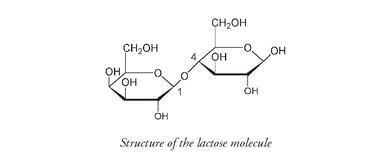
Galactose on the left is joined through C#1 to C#4 of glucose on the right.
Again, having an OH above or below the ring may seem like a very minor difference, but for those people who suffer from lactose intolerance, it is not. To digest lactose and other disaccharides or larger sugars, we need specific enzymes that initially break down these complex molecules into simpler monosaccharides. In the case of lactose, the enzyme is called
lactase
and is present in only small amounts in some adults. (Children generally produce greater amounts of lactase than adults.) Insufficient lactase makes the digestion of milk and milk products difficult and causes the symptoms associated with lactose intolerance: abdominal bloating, cramps, and diarrhea. Lactose intolerance is an inherited trait, easily treated with over-the-counter preparations of the lactase enzyme. Adults and children (but not babies) from certain ethnic groups, such as some African tribes, are missing the lactase enzyme completely. For these people, powdered milk and other milk products, often found in food aid programs, are indigestible and even harmful.
lactase
and is present in only small amounts in some adults. (Children generally produce greater amounts of lactase than adults.) Insufficient lactase makes the digestion of milk and milk products difficult and causes the symptoms associated with lactose intolerance: abdominal bloating, cramps, and diarrhea. Lactose intolerance is an inherited trait, easily treated with over-the-counter preparations of the lactase enzyme. Adults and children (but not babies) from certain ethnic groups, such as some African tribes, are missing the lactase enzyme completely. For these people, powdered milk and other milk products, often found in food aid programs, are indigestible and even harmful.
The brain of a normal healthy mammal uses only glucose for fuel. Brain cells are dependent on a minute-to-minute supply from the bloodstream, as there are essentially no fuel reserves or storage in the brain. If blood glucose level falls to 50 percent of the normal level, some symptoms of brain dysfunction appear. At 25 percent of the normal level, possibly from an overdose of insulinâthe hormone that maintains the level of glucose in the bloodâa coma may result.
SWEET TASTEWhat makes all these sugars so appealing is that they taste sweet, and humans like sweetness. Sweetness is one of the four principal tastes; the other three are sourness, bitterness, and saltiness. Achieving the ability to distinguish among these tastes was an important evolutionary step. Sweetness generally implies “good to eat.” A sweet taste indicates that fruit is ripe, whereas sour tells us there are still lots of acids present, and the unripe fruit may cause a stomachache. A bitter taste in plants often indicates the presence of a type of compound known as an alkaloid. Alkaloids are often poisonous, sometimes in only very small amounts, so the ability to detect traces of an alkaloid is a distinct advantage. It has even been suggested that the extinction of the dinosaurs might have been due to their inability to detect the poisonous alkaloids found in some of the flowering plants that evolved toward the end of the Cretaceous period, about the time the dinosaurs disappeared, although this is not the generally accepted theory of dinosaur extinction.
Humans do not seem to have an inborn liking for bitterness. In fact, their preference is probably just the opposite. Bitterness invokes a response involving secretion of extra saliva. This is a useful reaction to something poisonous in the mouth, allowing one to spit it out as completely as possible. Many people do, however, learn to appreciate, if not like, the bitter taste. Caffeine in tea and coffee and quinine in tonic water are examples of this phenomenon, although many of us still rely on having sugar in these drinks. The term
bittersweet,
connoting pleasure mixed with sadness, conveys our ambivalence about bitter tastes.
bittersweet,
connoting pleasure mixed with sadness, conveys our ambivalence about bitter tastes.
Our sense of taste is located in the taste buds, specialized groups of cells found mainly on the tongue. Not all parts of the tongue detect taste the same way or to the same degree. The front tip of the tongue is the most sensitive to sweetness, while sourness is detected most strongly on the sides of the tongue toward the back. You can test this easily for yourself by touching a sugar solution to the side of the tongue and then to the tip of the tongue. The tip of the tongue will definitely detect the sweet sensation more strongly. If you try the same thing with lemon juice, the result will be even more obvious. Lemon juice on the very tip of the tongue does not seem very sour, but put a freshly cut slice of lemon on the side of the tongue, and you will discover where the sourness reception area is the strongest. You can continue this experiment: bitterness is detected most strongly on the middle of the tongue but back from the tip, and the salty sensation is greatest just to each side of the tip.
Sweetness has been investigated far more than any of the other tastes, no doubt because, as in the days of the slave trade, it is still big business. The relationship between chemical structure and sweetness is complicated. One simple model, known as the A-H,B Model, suggests that a sweet taste depends on an arrangement of a group of atoms within a molecule. These atoms (A and B in the diagram) have a particular geometry, allowing atom B to be attracted to the hydrogen atom attached to atom A. This results in the short-term binding of the sweet molecule to a protein molecule of a taste receptor, causing a generation of a signal (transmitted through nerves) informing the brain, “This is sweet.” A and B are usually oxygen or nitrogen atoms, although one of them may also be a sulfur atom.
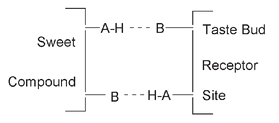
The A-H,B Model of Sweetness
There are many sweet compounds other than sugar, and not all of them are good to eat. Ethylene glycol, for example, is the major component of antifreeze used in car radiators. The solubility and flexibility of the ethylene glycol molecule, as well as the distance between its oxygen atoms (similar to the distance between oxygen atoms in sugars), account for its sweet taste. But it is very poisonous. A dose of as little as one tablespoon can be lethal for humans or family pets.
Interestingly, it is not ethylene glycol but what the body turns it into that is the toxic agent. Oxidation of ethylene glycol by enzymes in the body produces oxalic acid.

Oxalic acid occurs naturally in a number of plants, including some that we eat, such as rhubarb and spinach. We usually consume these foods in moderate amounts, and our kidneys can cope with the traces of oxalic acid from such sources. But if ethylene glycol is swallowed, the sudden appearance of a large amount of oxalic acid can cause kidney damage and death. Eating spinach salad and rhubarb pie at the same meal will not hurt you. It would probably be difficult to consume enough spinach and rhubarb to do any harm, except perhaps if you are prone to kidney stones, which build up over some years. Kidney stones consist mainly of calcium oxalate, the insoluble calcium salt of oxalic acid; those prone to kidney stones are often advised to avoid foods high in oxalates. For the rest of us, moderation is the best advice.
A compound that has a very similar structure to ethylene glycol and also tastes sweet is glycerol, but glycerol in moderate amounts is safe to consume. It is used as an additive in many prepared foods because of its viscosity and high water solubility. The term
food additive
has had a bad press in recent years, implying that food additives are essentially nonorganic, unhealthy, and unnatural. Glycerol is definitely organic, is nontoxic, and occurs naturally in products such as wine.
food additive
has had a bad press in recent years, implying that food additives are essentially nonorganic, unhealthy, and unnatural. Glycerol is definitely organic, is nontoxic, and occurs naturally in products such as wine.
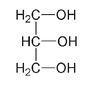
Glycerol
When you swirl a glass of wine, the “legs” that form on the glass are due to the presence of glycerol increasing the viscosity and smoothness characteristic of good vintages.
Other books
Crónica de una muerte anunciada by Gabriel García Márquez
20.000 leguas de viaje submarino by Julio Verne
Blissful volume 2 (New Adult Romance) by Wild, Clarissa
Ghost of the Chattering Bones by Gertrude Chandler Warner
The Soldier's Lady by Silver, Jordan
Criminal Love: A Billionaire Romance by Hodge, Monique
Voices In The Evening by Natalia Ginzburg
Drifters' Alliance, Book 2 by Elle Casey
Black Rabbit and Other Stories by Salvatore Difalco