Penny le Couteur & Jay Burreson (15 page)
Read Penny le Couteur & Jay Burreson Online
Authors: Napoleon's Buttons: How 17 Molecules Changed History
Tags: #Philosophy & Social Aspects, #Science, #General, #World, #Chemistry, #Popular Works, #History

Â
Â
Both the α polymer of glucose (starch) and the β polymer (cellulose) are essential components of our diet and as such have had, and always will have, an indispensable function in human society. But it is the non-dietary roles of cellulose and its various derivatives that have created milestones in history. Cellulose, in the form of cotton, was responsible for two of the most influential events of the nineteenth century: the Industrial Revolution and the American Civil War. Cotton was the star of the Industrial Revolution, transforming the face of England through rural depopulation, urbanization, rapid industrialization, innovation and invention, social change, and prosperity. Cotton evoked one of the greatest crises in the history of the United States; slavery was the most important issue in the Civil War between abolitionist North and the southern states, whose economic system was based on slave-grown cotton.
Nitrocellulose (guncotton) was one of the very first explosive organic molecules made by man, and its discovery marked the start of a number of modern industries originally based on nitrated forms of cellulose: explosives, photography, and the movie business. The synthetic textile industry, with its beginnings from rayonâa different form of celluloseâhas played a significant role in shaping the economy over the last century. Without these applications of the cellulose molecule, our world would be a very different place.
5. NITRO COMPOUNDS
S
CHÃNBEIN'S WIFE'S exploding apron was not the first example of a man-made explosive molecule, nor would it be the last. When chemical reactions are very rapid, they can have an awesome power. Cellulose is only one of the many molecules we have altered to take advantage of the capacity for explosive reaction. Some of these compounds have been of enormous benefit; others have caused widespread destruction. Through their very explosive properties, these molecules have had a marked effect on the world.
CHÃNBEIN'S WIFE'S exploding apron was not the first example of a man-made explosive molecule, nor would it be the last. When chemical reactions are very rapid, they can have an awesome power. Cellulose is only one of the many molecules we have altered to take advantage of the capacity for explosive reaction. Some of these compounds have been of enormous benefit; others have caused widespread destruction. Through their very explosive properties, these molecules have had a marked effect on the world.
Although the structures of explosive molecules vary widely, most often they contain a nitro group. This small combination of atoms, one nitrogen and two oxygens, NO
2
, attached at the right position, has vastly increased our ability to wage war, changed the fate of nations, and literally allowed us to move mountains.
GUNPOWDER-THE FIRST EXPLOSIVE2
, attached at the right position, has vastly increased our ability to wage war, changed the fate of nations, and literally allowed us to move mountains.
Gunpowder (or black powder), the first explosive mixture ever invented, was used in ancient times in China, Arabia, and India. Early Chinese texts refer to “fire-chemical” or “fire-drug.” Its ingredients were not recorded until early in A.D. 1000, and even then the actual proportions required of the component nitrate salt, sulfur, and carbon were not given. Nitrate salt (called saltpeter or “Chinese snow”) is potassium nitrate, chemical formula KNO
3
. The carbon in gunpowder was in the form of wood charcoal and gives the powder its black color.
3
. The carbon in gunpowder was in the form of wood charcoal and gives the powder its black color.
Gunpowder was initially used for firecrackers and fireworks, but by the middle of the eleventh century flaming objectsâused as weapons and known as fire arrowsâwere launched by gunpowder. In 1067 the Chinese placed the production of sulfur and saltpeter under government control.
We have no certainty as to when gunpowder arrived in Europe. The Franciscan monk Roger Bacon, born in England and educated at Oxford University and the University of Paris, wrote of gunpowder around 1260, a number of years before Marco Polo's return to Venice with stories of gunpowder in China. Bacon was also a physician and an experimentalist, knowledgeable in the sciences that we would now call astronomy, chemistry, and physics. He was also fluent in Arabic, and it is likely that he learned about gunpowder from a nomadic tribe, the Saracens, who acted as middlemen between the Orient and the West. Bacon must have been aware of the destructive potential of gunpowder, as his description of its composition was in the form of an anagram that had to be deciphered to reveal the ratio: seven parts saltpeter, five parts charcoal, and five parts sulfur. His puzzle remained unsolved for 650 years before finally being decoded by a British army colonel. By then gunpowder had, of course, been in use for centuries.
Present-day gunpowder varies somewhat in composition but contains a larger proportion of saltpeter than Bacon's formulation. The chemical reaction for the explosion of gunpowder can be written as
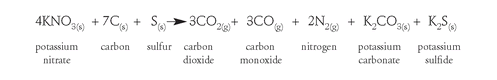
This chemical equation tells us the ratios of substances reacting and the ratios of the products obtained. The subscript (s) means the substance is a solid, and (g) means it is a gas. You can see from the equation that all the reactants are solids, but eight molecules of gases are formed: three carbon dioxide, three carbon monoxide, and two nitrogens. It is the hot, expanding gases produced from the rapid burning of gunpowder that propel a cannonball or bullet. The solid potassium carbonate and sulfide formed are dispersed as tiny particles, the characteristic dense smoke of exploding gunpowder.
Thought to have been produced somewhere around 1300 to 1325, the first firearm, the firelock, was a tube of iron loaded with gunpowder, which was ignited by the insertion of a heated wire. As more sophisticated firearms developed (the musket, the flintlock, the wheellock), the need for different rates of burning of gunpowder became apparent. Sidearms needed faster-burning powder, rifles a slower-burning powder, and cannons and rockets an even slower burn. A mixture of alcohol and water was used to produce a powder that caked and could be crushed and screened to give fine, medium, and coarse fractions. The finer the powder, the faster the burn, so it was possible to manufacture gunpowder that was appropriate for the various applications. The water used for manufacture was frequently supplied as urine from workers in the gunpowder mill; the urine of a heavy wine drinker was believed to create particularly potent gunpowder. Urine from a clergyman, or better yet a bishop, was also considered to give a superior product.
EXPLOSIVE CHEMISTRYThe production of gases and their consequent fast expansion from the heat of the reaction is the driving force behind explosives. Gases have a much greater volume than do similar amounts of solids or liquids. The destructive power of an explosion is due to the shock wave caused by the very rapid increase in volume as gases form. The shock wave for gunpowder travels around a hundred meters per second, but for “high” explosives (TNT or nitroglycerin, for example) it can be up to six thousand meters per second.
All explosive reactions give off large amounts of heat. Such reactions are said to be highly exothermic. The large amounts of heat act dramatically to increase the volume of the gasesâthe higher the temperature the larger the volume of gas. Heat comes from the energy difference between the molecules on each side of the explosive reaction equation. The molecules produced (on the right of the equation) have less energy tied up in their chemical bonds than the starting molecules (on the left). The compounds that form are more stable. In explosive reactions of nitro compounds, the extremely stable nitrogen molecule, N
2
, is formed. The stability of the N
2
molecule is due to the strength of the triple bond that holds the two nitrogen atoms together.
2
, is formed. The stability of the N
2
molecule is due to the strength of the triple bond that holds the two nitrogen atoms together.

Structure of the N
2
molecule
2
molecule
That this triple bond is very strong means that a lot of energy is needed to break it. Conversely, when the N
2
triple bond is made, a lot of energy is released, which is exactly what is wanted in an explosive reaction.
2
triple bond is made, a lot of energy is released, which is exactly what is wanted in an explosive reaction.
Besides production of heat and of gases, a third important property of explosive reactions is that they must be extremely rapid. If the explosive reaction were to occur slowly, the resulting heat would dissipate and the gases would diffuse into the surroundings without the violent pressure surge, damaging shock wave, and high temperatures characteristic of an explosion. The oxygen required for such a reaction has to come from the molecule that is exploding. It cannot come from the air, because oxygen from the atmosphere is not available quickly enough. Thus nitro compounds, in which nitrogen and oxygen are bonded together, are often explosive, while other compounds containing both nitrogen and oxygen, but not bonded together, are not.
This can be seen using isomers as an example, isomers being compounds that have the same chemical formula but different structures.
Para-
nitrotoluene and
para
-aminobenzoic acid both have seven carbon atoms, seven hydrogen atoms, one nitrogen atom, and two oxygen atoms for identical chemical formulae of C
7
H
7
NO
2
, but these atoms are arranged differently in each molecule.
Para-
nitrotoluene and
para
-aminobenzoic acid both have seven carbon atoms, seven hydrogen atoms, one nitrogen atom, and two oxygen atoms for identical chemical formulae of C
7
H
7
NO
2
, but these atoms are arranged differently in each molecule.
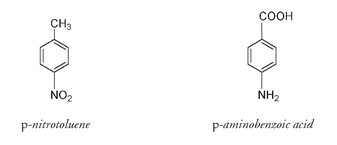
Para-
or
p
-nitrotoluene (the
para
just tells you that the CH
3
and NO
3
groups are at opposite ends of the molecule) can be explosive, whereas
p
-aminobenzoic acid is not at all explosive. In fact you have probably rubbed it over your skin in the summer; it is PABA, the active ingredient in many sunscreen products. Compounds such as PABA absorb ultraviolet light at the very wavelengths that have been found to be most damaging to skin cells. Absorption of ultraviolet light at particular wavelengths depends on the presence in the compound of alternating double and single bonds, possibly also with oxygen and nitrogen atoms attached. Variation in the number of bonds or atoms of this alternating pattern changes the wavelength of absorption. Other compounds that absorb at the required wavelengths can be used as sunscreens provided they also do not wash off easily in water, have no toxic or allergic effects, no unpleasant smell or taste, and do not decompose in the sun.
or
p
-nitrotoluene (the
para
just tells you that the CH
3
and NO
3
groups are at opposite ends of the molecule) can be explosive, whereas
p
-aminobenzoic acid is not at all explosive. In fact you have probably rubbed it over your skin in the summer; it is PABA, the active ingredient in many sunscreen products. Compounds such as PABA absorb ultraviolet light at the very wavelengths that have been found to be most damaging to skin cells. Absorption of ultraviolet light at particular wavelengths depends on the presence in the compound of alternating double and single bonds, possibly also with oxygen and nitrogen atoms attached. Variation in the number of bonds or atoms of this alternating pattern changes the wavelength of absorption. Other compounds that absorb at the required wavelengths can be used as sunscreens provided they also do not wash off easily in water, have no toxic or allergic effects, no unpleasant smell or taste, and do not decompose in the sun.
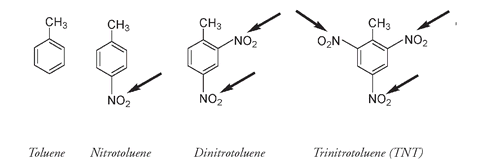
The explosiveness of a nitrated molecule depends on the number of nitro groups attached. Nitrotoluene has only one nitro group. Further nitration can add two or three more nitro groups, resulting in di- or trinitrotoluenes respectively. While nitrotoluene and dinitrotoluene can explode, they do not pack the same power as the high-explosive trinitrotoluene (TNT) molecule.
Other books
Descended (The Red Blindfold Book 2) by Rose Devereux
The Magician by Sol Stein
Under Orders by Dick Francis
Lye in Wait by Cricket McRae
Trouble Shooter (1974) by L'amour, Louis - Hopalong 04
Seeking Safety by Karen Ward
A Soldier's Story by Blair, Iona
Mayhem in Bath by Sandra Heath
Harlem Nocturne by Farah Jasmine Griffin
Apocalypse (The Wasteland Chronicles, #1) by Kyle West