Penny le Couteur & Jay Burreson (42 page)
Read Penny le Couteur & Jay Burreson Online
Authors: Napoleon's Buttons: How 17 Molecules Changed History
Tags: #Philosophy & Social Aspects, #Science, #General, #World, #Chemistry, #Popular Works, #History

Most countries now ban the importation, manufacture, or possession of heroin. But this has done little to stop the illegal trade in this molecule. Laboratories that are set up to manufacture heroin from morphine often have a major problem disposing of acetic acid, one of the side products of the acylation reaction. Acetic acid has a very distinctive smell, that of vinegar, which is a 4 percent solution of this acid. This smell often alerts authorities to the existence of an illicit heroin manufacturer. Specially trained police dogs can detect faint traces of vinegar odor below the level of human sensitivity.
Investigation into why morphine and similar alkaloids are such effective pain relievers suggests that morphine does not interfere with nerve signals to the brain. Instead it selectively changes how the brain receives these messagesâthat is, how the brain perceives the pain being signaled. The morphine molecule seems able to occupy and block a pain receptor in the brain, a theory that correlates with the idea that a certain shape of chemical structure is needed to fit into a pain receptor.
Morphine mimics the action of endorphins, compounds found in very low concentrations in the brain that serve as natural pain relievers and that increase in concentration in times of stress. Endorphins are polypeptides, compounds made from joining amino acids together, end to end. This is the same peptide formation that is responsible for the structure of proteins such as silk (see Chapter 6). But whereas a silk molecule has hundreds or even thousands of amino acids, endorphins consist of only a few. Two endorphins that have been isolated are pentapeptides, meaning that they contain five amino acids. Both of these endorphin pentapeptides and morphine have a structural feature in common: they all contain a β-phenylethylamine unit, the same chemical construction that is thought responsible for affecting the brain in LSD, in mescaline, and in some other hallucinogenic molecules.
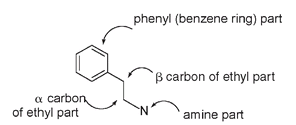
The
β
-phenylethylamine unit
β
-phenylethylamine unit
Though the pentapeptide endorphin molecules are otherwise quite unlike the morphine molecule, this structural similarity is thought to account for the common binding site in the brain.
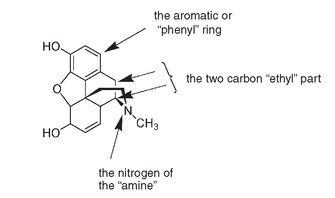
Structure of the morphine molecule, showing the
β
-phenylethylamine unit
β
-phenylethylamine unit
But morphine and its analogs differ in biological activity from other hallucinogens in that they also have narcotic effectsâpain-relief, sleep-inducing, and addictive components. These are thought to be due to another combination found in the chemical structure of, taken in order: (1) a phenyl or aromatic ring, (2) a quaternary carbon atom; that is, a carbon atom directly attached to four other carbon atoms, (3) a CH
2
-CH
2
group attached to (4) a tertiary N atom (a nitrogen atom directly attached to three other carbon atoms).
2
-CH
2
group attached to (4) a tertiary N atom (a nitrogen atom directly attached to three other carbon atoms).
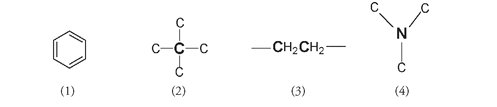
(1) The benzene ring, (2) quaternary carbon atom (bolded), (3) the two CH
2
groups with the carbons bolded, and (4) the tertiary nitrogen atom (bolded)
2
groups with the carbons bolded, and (4) the tertiary nitrogen atom (bolded)
Combined, this set of requirementsâknown as the morphine ruleâlooks like:
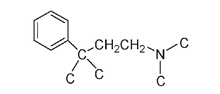
Essential components for the morphine rule
You can see in the diagrams of morphine that all four requirements are present, as they also are in codeine and heroin.
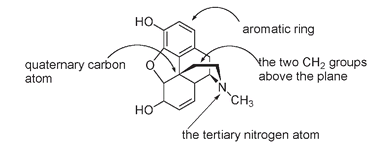
Morphine structure, showing how it fits the morphine rule for biological activit.
The discovery that this part of the molecule might account for narcotic activity is another example of serendipity in chemistry. Investigators injecting a man-made compound, meperidine, into rats noted that it caused them to hold their tails in a certain way, an effect previously seen with morphine.
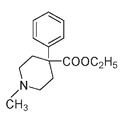
Meperidine
The meperidine molecule was not particularly similar to the morphine molecule. What meperidine and morphine did have in common were (1) an aromatic or phenyl ring attached to (2) a quaternary carbon, followed by (3) the CH
2
-CH
2
group and then a tertiary nitrogen; in other words, the same arrangement that was to become known as the morphine rule.
2
-CH
2
group and then a tertiary nitrogen; in other words, the same arrangement that was to become known as the morphine rule.
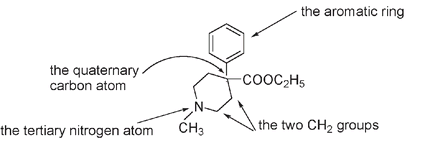
Highlighting the morphine rule for the meperidine or Demerol structure
Testing of meperidine revealed that it had analgesic properties. Usually known by the trade name Demerol, it is often used instead of morphine as, although it is less effective, it is unlikely to cause nausea. But it is still addictive. Another synthetic and very potent analgesic, methadone, like heroin and morphine, depresses the nervous system but does not produce the drowsiness or the euphoria associated with opiates. The structure of methadone is not a complete match to the requirements for the morphine rule. There is a CH
3
group attached to the second carbon atom of the -CH
2
-CH
2
-. This very small change in structure is presumed responsible for the difference in biological activity.
3
group attached to the second carbon atom of the -CH
2
-CH
2
-. This very small change in structure is presumed responsible for the difference in biological activity.
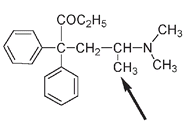
The structure of methadone. The arrow shows the position of the CH
3
group, the only deviation from the morphine rule but enough to change the physiological effect.
3
group, the only deviation from the morphine rule but enough to change the physiological effect.
Methadone is, however, still addictive. Dependence on heroin can be transferred to dependence on methadone, but whether this is a reasonable method of dealing with the problems associated with heroin addiction is still a subject of debate.
DRINKING SMOKENicotine, the second alkaloid associated with the Opium Wars, was unknown in Europe when Christopher Columbus landed in the New World. There he saw both men and women who “drank” or inhaled the smoke of rolls of burning leaves inserted into their nostrils. Smoking, snuffing (inhaling a powdered form into the nose), and chewing leaves of tobacco plants, species of the genus
Nicotiana,
was widespread among the Indians of South America, Mexico, and the Caribbean. The use of tobacco was mainly ceremonial; tobacco smoke sucked from pipes or from rolled leaves, or inhaled directly from foliage strewn over glowing embers, was said to cause trances or hallucinations in participants. This would have meant that their tobacco had significantly higher concentrations of active ingredients than are found in the species
Nicotiana tabacum
that was introduced to Europe and the rest of the world. The tobacco noted by Columbus was most probably
Nicotiana rustica,
the tobacco of the Mayan civilization, which is known to be a more potent variety.
Nicotiana,
was widespread among the Indians of South America, Mexico, and the Caribbean. The use of tobacco was mainly ceremonial; tobacco smoke sucked from pipes or from rolled leaves, or inhaled directly from foliage strewn over glowing embers, was said to cause trances or hallucinations in participants. This would have meant that their tobacco had significantly higher concentrations of active ingredients than are found in the species
Nicotiana tabacum
that was introduced to Europe and the rest of the world. The tobacco noted by Columbus was most probably
Nicotiana rustica,
the tobacco of the Mayan civilization, which is known to be a more potent variety.
Other books
Angels at the Gate by T. K. Thorne
Survival Paranoia (Survival series) by Glass, Kay
The Center Cannot Hold: My Journey Through Madness by Elyn R. Saks
The Scandal and Carter O'Neill by Molly O’Keefe
A Touch in Time by McKenna Chase
Cry Mercy by Mariah Stewart
The Alington Inheritance by Wentworth, Patricia
The Scarlet Letters by Ellery Queen
Habit by Susan Morse
Hunter's Season: Elder Races, Book 4 by Thea Harrison