Penny le Couteur & Jay Burreson (19 page)
Read Penny le Couteur & Jay Burreson Online
Authors: Napoleon's Buttons: How 17 Molecules Changed History
Tags: #Philosophy & Social Aspects, #Science, #General, #World, #Chemistry, #Popular Works, #History

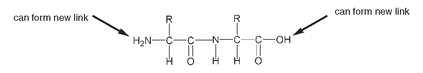
Of course, on one end of this new molecule there is still an OH that can be used to form another peptide bond with another amino acid, and on the other end there is an NH
2
(also written H
2
N) that can form a peptide bond to yet another amino acid.
2
(also written H
2
N) that can form a peptide bond to yet another amino acid.
The amide group
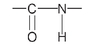 is usually shown in a more space-saving way as
is usually shown in a more space-saving way as
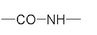

If we add two more amino acids, there are now four amino acids joined through amide linkages.
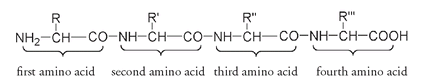

As there are four amino acids, there are four side groups, designated above as R, R', R" and R"'. These side groups could all be the same, or some of them could be the same, or they could all be different. Despite having only four amino acids in the chain, a very large number of combinations is possible. R could be any of twenty-two amino acids, R' could also be any of the twenty-two, and so could R" and R"'. This means there are 22
4
or 234,256 possibilities. Even a very small protein such as insulin, the hormone secreted by the pancreas that regulates glucose metabolism, contains 51 amino acids, so the number of combinations possible for insulin would be 22
51
(2.9 x 10
68
), or billions of billions.
4
or 234,256 possibilities. Even a very small protein such as insulin, the hormone secreted by the pancreas that regulates glucose metabolism, contains 51 amino acids, so the number of combinations possible for insulin would be 22
51
(2.9 x 10
68
), or billions of billions.
It is estimated that 80 to 85 percent of the amino acids of silk is a repeating sequence of glycine-serine-glycine-alanine-glycine-alanine. A chain of the silk protein polymer has a zigzag arrangement with the side groups alternating on each side.
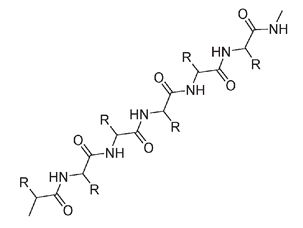
The silk protein chain is a zigzag; the R groups alternate on each side of the chain.
These chains of the protein molecule lie parallel with adjacent chains running in opposite directions. They are held to one another through cross-attractions between the molecular strands, as shown by dotted lines below.
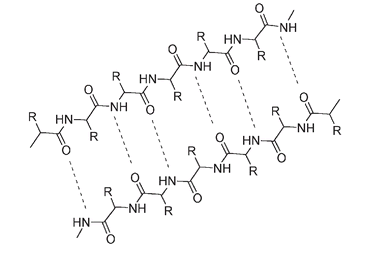
Attractions between side-by-side protein chains hold silk molecules together.
This produces a pleated sheet structure, where alternate R groups along the protein chain point either up or down. It can be shown as:
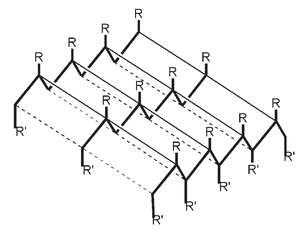
The pleated sheet structure. The bold lines represent the protein amino acid chains. Here R represents groups that are above the sheet, while R' groups (where shown) are below. The narrow and dotted lines show the attractive forces holding the protein chains together.
The flexible structure resulting from the pleated sheet structure is resistant to stretching and accounts for many of the physical properties of silk. The protein chains fit together tightly; the small R groups on the surfaces are relatively similar in size, creating a uniform surface responsible for the smooth feel of silk. As well, this uniform surface acts as a good reflector of light, accounting for silk's characteristic luster. Thus many of the highly valued qualities of silk are due to the small side groups in its protein structure.
Connoisseurs of silk also appreciate the fabric's “sparkle,” which is attributed to the fact that not all silk molecules are part of a regular pleated sheet structure. These irregularities break up reflected light, creating flashes of brightness. Often considered unsurpassed in its ability to absorb both natural and man-made dyes, silk is easy to color. This property is again due to the parts of the silk structure that are not included in the regular repeating sequence of pleated sheets. Among these remaining 15 to 20 percent or so of amino acidsâthose that are not glycine, alanine, or serineâare some whose side groups can easily chemically bond with dye molecules, producing the deep, rich, and colorfast hues for which silk is famous. It is this dual nature of silkâthe repetitive small-side-group pleated sheet structure responsible for strength, sheen, and smoothness, combined with the more variable remaining amino acids, giving sparkle and ease of dyeingâthat has for centuries made silk such a desirable fabric.
THE SEARCH FOR SYNTHETIC SILKAll of these properties make silk difficult to replicate. But because silk was so expensive and the demand for it so great, numerous attempts were made, beginning in the late nineteenth century, to produce a synthetic version. Silk is a very simple moleculeâjust a repetition of very similar units. But attaching these units together in the random and non-random combination found in natural silk is a very complicated chemical problem. Modern chemists are now able, on a very small scale, to replicate the set pattern of a particular protein strand, but the process is time consuming and exacting. A silk protein produced in the laboratory in this way would be many times more expensive than the natural article.
As the complexities of the chemical structure of silk were not understood until the twentieth century, early efforts to make a synthetic version were largely guided by fortunate accidents. Sometime in the late 1870s a French count, Hilaire de Chardonnet, while pursuing his favorite hobby of photography, discovered that a spilled solution of collodionâthe nitrocellulose material used to coat photographic platesâhad set to a sticky mass, from which he was able to pull out long threads resembling silk. This reminded Chardonnet of something he had seen a number of years previously: as a student, he had accompanied his professor, the great Louis Pasteur, to Lyons, in the south of France, to investigate a silkworm disease that was causing enormous problems for the French silk industry. Though unable to find the cause of the silkworm blight, Chardonnet had spent much time studying the silkworm and how it spun its silk fiber. With this in mind, he now tried forcing collodion solution through a set of tiny holes. He thus produced the first reasonable facsimile of silk fiber.
The words
synthetic
and
artificial
are often used interchangeably in everyday language and are given as synonyms in most dictionaries. But there is an important chemical distinction between them. For our purposes
synthetic
is taken to mean that a compound is man-made by chemical reactions. The product may be one that occurs in nature or it may not occur naturally. If it does occur in nature, the synthetic version will be chemically identical to that of the natural source. For example, ascorbic acid, vitamin C, can be synthesized in a laboratory or factory; synthetic vitamin C has exactly the same chemical structure as naturally occurring vitamin C.
synthetic
and
artificial
are often used interchangeably in everyday language and are given as synonyms in most dictionaries. But there is an important chemical distinction between them. For our purposes
synthetic
is taken to mean that a compound is man-made by chemical reactions. The product may be one that occurs in nature or it may not occur naturally. If it does occur in nature, the synthetic version will be chemically identical to that of the natural source. For example, ascorbic acid, vitamin C, can be synthesized in a laboratory or factory; synthetic vitamin C has exactly the same chemical structure as naturally occurring vitamin C.
The word
artificial
refers more to the properties of a compound. An artificial compound has a different chemical structure from that of another compound, but it has properties similar enough to mimic the other's role. For example, an artificial sweetener does not have the same structure as sugar, but it will have an important propertyâin this case, sweetnessâin common. Artificial compounds are often man-made and are therefore synthetic, but they need not necessarily be synthetic. Some artificial sweeteners are naturally occurring.
artificial
refers more to the properties of a compound. An artificial compound has a different chemical structure from that of another compound, but it has properties similar enough to mimic the other's role. For example, an artificial sweetener does not have the same structure as sugar, but it will have an important propertyâin this case, sweetnessâin common. Artificial compounds are often man-made and are therefore synthetic, but they need not necessarily be synthetic. Some artificial sweeteners are naturally occurring.
What Chardonnet produced was artificial silk, not synthetic silk, although it was made synthetically. (By our definitions, synthetic silk would be man-made but chemically identical to real silk.)
Chardonnet silk,
as it came to be known, resembled silk in some of its properties but not in all of them. It was soft and lustrous, but unfortunately it was highly flammableânot a desirable property for a fabric. Chardonnet silk was spun from a solution of nitrocellulose, and as we have seen, nitrated versions of cellulose are flammable and even explosive, depending on the degree of nitration of the molecule.
Chardonnet silk,
as it came to be known, resembled silk in some of its properties but not in all of them. It was soft and lustrous, but unfortunately it was highly flammableânot a desirable property for a fabric. Chardonnet silk was spun from a solution of nitrocellulose, and as we have seen, nitrated versions of cellulose are flammable and even explosive, depending on the degree of nitration of the molecule.
Other books
Cemetery World by Clifford D. Simak
Safekeeping by Jessamyn Hope
The Funny Man by John Warner
All Woman and Springtime by Brandon Jones
Between the Dark and the Daylight: Encountering and Embracing the Contradictions of Life by Joan Chittister, Osb, Joan Sister Chittister
Paying the Price (Book 5 of The Empire of Bones Saga) by Terry Mixon
97 segundos by Ángel Gutiérrez y David Zurdo
Sexy Book of Sexy Sex by Kristen Schaal
Stuff to Die For by Don Bruns
The Cauldron of Fear by Joe Dever