Penny le Couteur & Jay Burreson (46 page)
Read Penny le Couteur & Jay Burreson Online
Authors: Napoleon's Buttons: How 17 Molecules Changed History
Tags: #Philosophy & Social Aspects, #Science, #General, #World, #Chemistry, #Popular Works, #History

This fatty acid still has twelve carbon atoms.
When three molecules of water (H
2
O) are eliminated between the H from each of the three OH groups on glycerol and OH from the HOOC of three different fatty-acid molecules, a triglyceride molecule is formed. This condensation processâthe joining of molecules through the loss of H
2
Oâis similar to the formation of polysaccharides (discussed in Chapter 4).
2
O) are eliminated between the H from each of the three OH groups on glycerol and OH from the HOOC of three different fatty-acid molecules, a triglyceride molecule is formed. This condensation processâthe joining of molecules through the loss of H
2
Oâis similar to the formation of polysaccharides (discussed in Chapter 4).
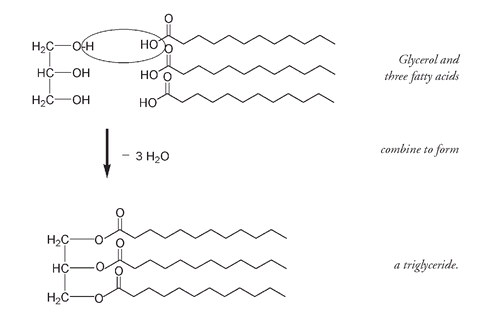
The triglyceride molecule shown above has all three fatty-acid molecules the same. But it is also possible for only two of the fatty-acid molecules to be the same. Or they can all be different. Fats and oils have the same glycerol portion; it is the fatty acids that vary. In the previous example we used what is known as a saturated fatty acid.
Saturated
in this case means saturated with hydrogen; no more hydrogen can be added to the fatty-acid portion of the molecule, as there are no carbon-to-carbon double bonds that can be broken to allow the attachment of more hydrogen atoms. If such bonds are present in the fatty acid, it is termed unsaturated. Some common saturated fatty acids are:
Saturated
in this case means saturated with hydrogen; no more hydrogen can be added to the fatty-acid portion of the molecule, as there are no carbon-to-carbon double bonds that can be broken to allow the attachment of more hydrogen atoms. If such bonds are present in the fatty acid, it is termed unsaturated. Some common saturated fatty acids are:
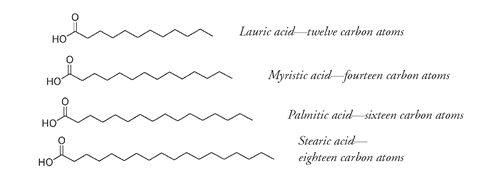
From their names it is not hard to guess that the main source of stearic acid is beef tallow and that palmitic acid is a component of palm oil.
Almost all fatty acids have an even number of carbon atoms. The examples above are the most common fatty acids, although others do exist. Butter contains butyric acid (named from butter), with only four carbon atoms, and caproic acid, also present in butter and in fat from goat's milkâ
caper
is Latin for goatâhas six carbon atoms.
caper
is Latin for goatâhas six carbon atoms.
Unsaturated fatty acids contain at least one carbon-to-carbon double bond. If there is only one of these double bonds, the acid is referred to as
monounsaturated;
with more than one double bond it is
polyunsaturated.
The triglyceride shown below is formed from two monounsaturated fatty acids and one saturated fatty acid. The double bonds are cis in arrangement, as the carbon atoms of the long chain are on the same side of the double bond.
monounsaturated;
with more than one double bond it is
polyunsaturated.
The triglyceride shown below is formed from two monounsaturated fatty acids and one saturated fatty acid. The double bonds are cis in arrangement, as the carbon atoms of the long chain are on the same side of the double bond.
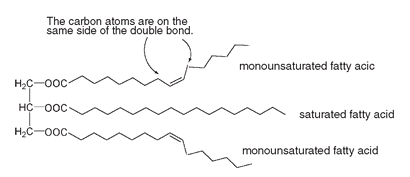
Triglyceride from two monounsaturated and one saturated fatty acid
This puts a kink in the chain, so such triglycerides cannot pack together as closely as triglycerides composed of saturated fatty acids (below).
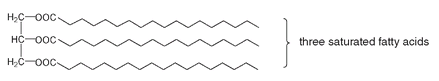
Triglyceride from three saturated fatty acids
The more double bonds in a fatty acid, the more bent it is and the less efficient its packing. Less efficient packing requires less energy to overcome the attractions holding the molecules together, and they can therefore be separated at lower temperatures. Triglycerides with a higher proportion of unsaturated fatty acids tend to be liquids at room temperature rather than solids. We call them oils; they are most often of plant origin. Saturated fatty acids that can pack closely together require more energy to separate individual molecules and so melt at higher temperatures. Triglycerides from animal sources, with a higher proportion of saturated fatty acids than oils, are solid at room temperature. We call them fats.
Some common unsaturated fatty acids are:
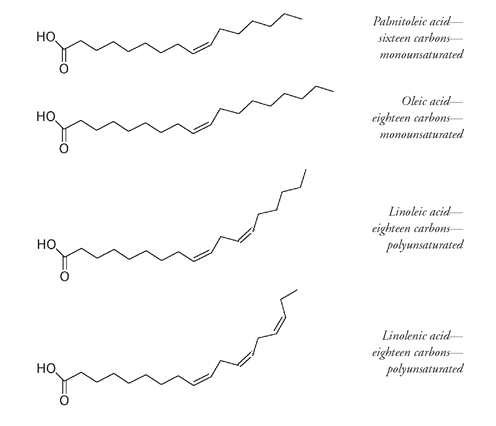
The monounsaturated, eighteen-carbon oleic acid is the major fatty acid of olive oil. Although oleic acid is found in other oils and also in many fats, olive oil is the most important source of this fatty acid. Olive oil contains a larger proportion of monounsaturated fatty acid than any other oil. The percentage of oleic acid in olive oil varies from about 55 to 85 percent, depending on the variety and the growing conditions, cooler areas producing a higher oleic acid content than warmer areas. There is now convincing evidence that a diet with a high proportion of saturated fat can contribute to the development of heart disease. The incidence of heart disease is lower in the Mediterranean region, where a lot of olive oilâand oleic acidâis consumed. Saturated fats are known to increase serum cholesterol concentrations, whereas polyunsaturated fats and oils lower these levels. Monounsaturated fatty acids, like oleic acid, have a neutral effect on the serum cholesterol level (the level of cholesterol in the blood).
The relationship between heart disease and fatty acids also involves another factor: the ratio of high-density lipoprotein (known as HDL) to low-density lipoprotein (known as LDL). A lipoprotein is a water-insoluble accumulation of cholesterol, protein, and triglycerides. High-density lipoproteinsâoften called the “good” lipoproteinsâtransport cholesterol from cells that have accumulated too much of this compound back to the liver for disposal. This prevents excess cholesterol from depositing on artery walls. The “bad” lipoproteins, LDLs, transport cholesterol from the liver or small intestine to newly formed or growing cells. While this is a necessary function, too much cholesterol in the bloodstream ultimately ends up as deposits of plaque on the artery walls, leading to narrowing of the arteries. If the coronary arteries leading to the heart muscles become clogged, the resultant decreased blood flow can cause chest pain and heart attacks.
It is the ratio of HDL to LDL, as well as the total cholesterol level, that is important in determining the risk of heart disease. Although polyunsaturated triglycerides have the positive effect of reducing serum cholesterol levels, they also lower the HDL:LDL ratio, a negative effect. Monounsaturated triglycerides like olive oil, while not reducing serum cholesterol levels, increase the HDL:LDL ratio, that is, the proportion of good lipoprotein to bad lipoprotein. Among the saturated fatty acids, palmitic (C
16
) and lauric (C
12
) acids raise LDL levels appreciably. The so-called tropical oilsâcoconut, palm, and palm kernelâwhich have high proportions of these fatty acids, are particularly suspect in heart disease because they increase both serum cholesterol and LDL levels.
16
) and lauric (C
12
) acids raise LDL levels appreciably. The so-called tropical oilsâcoconut, palm, and palm kernelâwhich have high proportions of these fatty acids, are particularly suspect in heart disease because they increase both serum cholesterol and LDL levels.
Although the healthy properties of olive oil were prized by ancient Mediterranean societies and were considered to account for longevity, there was no knowledge of the chemistry behind these beliefs. In fact, in times when the main dietary problem would have been simply to obtain enough calories, serum cholesterol levels and HDL:LDL ratios would have been irrelevant. For centuries, for the vast majority of the population of northern Europe, where the main source of triglycerides in the diet was animal fat and life expectancy was less than forty years, hardening of the arteries was not a problem. Only with increased life expectancy and the higher intake of saturated fatty acids accompanying economic prosperity has coronary heart disease become a major cause of death.
Another aspect of the chemistry of olive oil also accounts for its importance in the ancient world. As the number of carbon-to-carbon double bonds in a fatty acid increases, so does the tendency for the oil to oxidizeâbecome rancid. The proportion of polyunsaturated fatty acids in olive oil is much lower than in other oils, usually less than 10 percent, giving olive oil a longer shelf life than almost any other oil. As well, olive oil contains small amounts of polyphenols and of vitamins E and K, antioxidant molecules that play a critical role as natural preservatives. The traditional cold-press method of extracting oil from olives helps retain these antioxidant molecules, which can be destroyed by high temperatures.
Today one method of improving the stability and increasing the shelf life of oils is the elimination of some of the double bonds by hydrogenation, a process of adding hydrogen atoms to the double bonds of unsaturated fatty acids. The result is also a more solid triglyceride; this is the method used to convert oils into butter substitutes like margarine. But the process of hydrogenation also changes the remaining double bonds from the cis configuration to the trans configuration, where the carbon atoms of the chain are on opposite sides of the double bond.
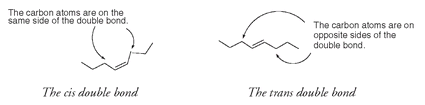
Trans-fatty acids are known to elevate LDL levels but not as much as saturated fatty acids.
TRADE IN OLIVE OILThe natural preservatives present as antioxidants in olives would have been of paramount importance to the oil traders of ancient Greece. This civilization was a loose association of city-states, with a common language, a common culture, and a common agricultural economic base: wheat, barley, grapes, figs, and olives. For centuries the land around the shores of the Mediterranean Sea was more wooded than it is now; the soil was more fertile, and more water was available from springs. As the population grew, cultivation of crops spread from the original small valleys up the sides of coastal mountains. With its ability to grow on steep and stony ground and withstand drought, the olive tree became increasingly more important. Its oil was even more valuable as an export commodity, for in the sixth century B.C., along with the strict laws against uncontrolled felling of olive trees, Solon of Athens also mandated olive oil as the only agricultural product that could be exported. As a result, coastal forests were cut down and more olive trees were planted. Where grain crops once grew, olive groves thrived.
Other books
Ready For You by J. L. Berg
Whirl by M, Jessie
The Willows by Mathew Sperle
Lady Afraid by Lester Dent
Crooked Kingdom by Leigh Bardugo
The Blackstone Commentaries by Rob Riggan
L. A. Candy by Lauren Conrad
Harmony 03 After Glow by Jayne Castle
A Million Guilty Pleasures: Million Dollar Duet by C.L. Parker