Penny le Couteur & Jay Burreson (43 page)
Read Penny le Couteur & Jay Burreson Online
Authors: Napoleon's Buttons: How 17 Molecules Changed History
Tags: #Philosophy & Social Aspects, #Science, #General, #World, #Chemistry, #Popular Works, #History

The use of tobacco spread quickly throughout Europe, and tobacco cultivation soon followed. Jean Nicot, the French ambassador to Portugal whose name is commemorated in the botanical name of the plant and the name of the alkaloid, was a tobacco enthusiast, as were other notable sixteenth-century figures: Sir Walter Raleigh in England and Catherine de Médicis, Queen of France. Smoking did not, however, meet with universal approval. Papal edicts banned the use of tobacco in church, and King James I of England is said to have authored a 1604 pamphlet decrying the “custome loathesome to the eye, hatefull to the nose, harmefull to the brain and daungerous to the lungs.”

An engraving from Brazil (around 1593) is the first copperplate showing smoking in South America. A plant is smoked through a long tube at this Tupi Indian feast.
(Courtesy of John G. Lord Collection)
(Courtesy of John G. Lord Collection)
In 1634 smoking was outlawed in Russia. Punishment for breaking this law was extremely harshâslitting of the lips, flogging, castration, or exile. Around fifty years later the ban was removed as Tsar Peter the Great, a smoker, promoted the use of tobacco. Just as Spanish and Portuguese sailors took chili peppers containing the capsaicin alkaloid around the world, so they introduced tobacco and the nicotine alkaloid to every port they visited. By the seventeenth century tobacco smoking had become widespread throughout the East, and draconian penalties, including torture, did little to stop its popularity. Although various countries, including Turkey, India, and Persia, at times prescribed the ultimate cure for addiction to tobaccoâthe death penaltyâsmoking is just as widespread in these places today as anywhere else.
From the beginning the supply of tobacco cultivated in Europe could not meet the demand. Spanish and English colonies in the New World soon started growing tobacco for export. Tobacco cultivation was highly labor intensive; weeds had to be kept under control, tobacco plants trimmed to the right height, suckers pruned, pests removed, and leaves manually harvested and prepared for drying. This work, done on plantations mainly by slaves, means that nicotine joins glucose, cellulose, and indigo as another molecule involved in slavery in the New World.
There are at least ten alkaloids in tobacco, the major one being nicotine. The content of nicotine in tobacco leaves varies from 2 to 8 percent, depending on the method of culture, climate, soil, and the process used to cure the leaves. In very small doses nicotine is a stimulant of the central nervous system and the heart, but eventually, or with larger doses, it acts as a depressant. This apparent paradox is explained by nicotine's ability to imitate the role of a neurotransmitter.
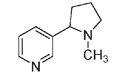
The structure of nicotine
The nicotine molecule forms a bridge at the junction between nerve cells, which initially heightens the transmission of a neurological impulse. But this connection is not readily cleared between impulses, so eventually the transmission site becomes obstructed. The stimulating effect of nicotine is lost, and muscle activity, particularly the heart, is slowed. Thus the blood circulation slows, and oxygen is delivered to the body and the brain at a lower rate, resulting in an overall sedative effect. This accounts for nicotine users who speak of needing a cigarette to calm their nerves, but nicotine is actually counterproductive in situations where an alert mind is required. As well, longtime tobacco users are more susceptible to infections such as gangrene that thrive in the low oxygen conditions from poor circulation.
In larger doses nicotine is a lethal poison. Absorbing a dose as small as fifty milligrams can kill an adult in just a few minutes. But its toxicity depends not only on amount but also on how the nicotine enters the body. Nicotine is about a thousand times more potent when absorbed through the skin as when taken orally. Stomach acids presumably break down the nicotine molecule to some extent. In smoking much of the alkaloid content in tobacco is oxidized to less toxic products by the high temperature of burning. This does not mean tobacco smoking is harmless, just that if this oxidation of most of the nicotine and other tobacco alkaloids did not occur, smoking would invariably be fatal with only a few cigarettes. As it is, the nicotine that remains in tobacco smoke is particularly hazardous, being absorbed directly from the lungs into the bloodstream.
Nicotine is a potent natural insecticide. Many millions of pounds of nicotine were produced for use as an insecticide in the 1940s and 1950s before synthetic pesticides were developed. Yet nicotinic acid and pyridoxine, with similar structures to nicotine, are not poisons. They are, in fact, beneficialâthey are both B vitamins, essential nutrients for our health and survival. Once again a small change in chemical structure makes an enormous difference in properties.
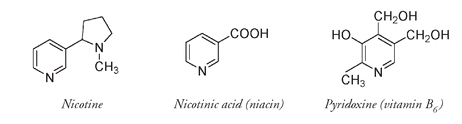
In humans a dietary deficiency of nicotinic acid (also known as niacin) results in the disease pellagra, which is characterized by a set of three symptoms: dermatitis, diarrhea, and dementia. It is prevalent where the diet is almost entirely composed of corn and originally was thought to be an infectious disease, possibly a form of leprosy. Until pellagra was identified as caused by a lack of niacin, many of its victims were institutionalized in insane asylums. Pellagra was common in the southern United States in the early part of the twentieth century, but efforts by Joseph Goldberger, a doctor with the U.S. Public Health Service, convinced the medical community that it was indeed a deficiency disease. The name
nicotinic acid
was changed to
niacin
when commercial bakers did not want their vitamin-enriched white bread to bear a name that sounded too similar to nicotine.
THE STIMULATING STRUCTURE OF CAFFEINEnicotinic acid
was changed to
niacin
when commercial bakers did not want their vitamin-enriched white bread to bear a name that sounded too similar to nicotine.
Caffeine, the third alkaloid connected to the Opium Wars, is also a psychoactive drug, but it is freely available almost everywhere in the world and is unregulated to such an extent that drinks laden with extra caffeine are manufactured and advertised as such. The structures of caffeine and the very closely related alkaloids theophylline and theobromine are shown below.
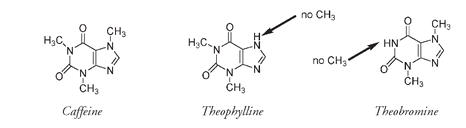
Theophylline, found in tea, and theobromine, in cocoa, differ from caffeine only in the number of CH
3
groups attached to the rings of the structure; caffeine having three and theophylline and theobromine each having two but in slightly different positions. This very small change of molecular structure accounts for the different physiological effect of these molecules. Caffeine is found naturally in coffee beans, tea leaves, and to a lesser extent cacao pods, cola nuts, and other plant sources mainly from South America, such as maté leaves, guarana seeds, and yoco bark.
3
groups attached to the rings of the structure; caffeine having three and theophylline and theobromine each having two but in slightly different positions. This very small change of molecular structure accounts for the different physiological effect of these molecules. Caffeine is found naturally in coffee beans, tea leaves, and to a lesser extent cacao pods, cola nuts, and other plant sources mainly from South America, such as maté leaves, guarana seeds, and yoco bark.
Caffeine is a powerful central nervous stimulant and one of the most studied drugs in the world. The latest of numerous theories that have been suggested over the years to explain its effects on human physiology is that caffeine blocks the effect of adenosine in the brain and in other parts of the body. Adenosine is a neuromodulator, a molecule that decreases the rate of spontaneous nerve firing and thus slows the release of other neurotransmitters; therefore it can induce sleep. Caffeine cannot be said to wake us up, although it may feel like it does; its effect is really to hinder the normal role of adenosine in making us sleepy. When caffeine occupies adenosine receptors in other parts of the body, we experience a caffeine buzz: heartbeat rate increases, some blood vessels constrict while others open, and certain muscles more easily contract.
Caffeine is used medicinally to relieve and prevent asthma, to treat migraines, to increase blood pressure, as a diuretic, and for a host of other conditions. It is often found in both over-the-counter and prescription medications. Numerous studies have looked for possible negative side effects of caffeine, including its relationship to various forms of cancer, heart disease, osteoporosis, ulcers, liver disease, premenstrual syndrome, kidney disease, sperm motility, fertility, fetal development, hyperactivity, athletic performance, and mental dysfunction. So far there is no clear evidence that any of these can be linked to moderate amounts of caffeine consumption.
But caffeine is toxic; a fatal dose is estimated at about ten grams taken orally by an average-sized adult. Since the caffeine content for a cup of coffee varies between 80 to 180 milligrams, depending on the method of preparation, you would have to drink something like 55 to 125 cups, all at one time, in order to receive a lethal dose. Obviously, caffeine poisoning by this method is most unlikely, if not absolutely impossible. By dry weight, tea leaves have twice as much caffeine as coffee beans, but because less tea is used per cup and less caffeine is extracted by the normal method of making tea, a cup of tea ends up with about half the caffeine of a cup of coffee.
Tea also contains small amounts of theophylline, a molecule that has a similar effect to caffeine. Theophylline is widely used today in the treatment of asthma. It is a better bronchodilator, or bronchial tissue relaxant, than caffeine, while having less of an effect on the central nervous system. The cacao pod, the source of cocoa and chocolate, contains 1 to 2 percent of theobromine. This alkaloid molecule stimulates the central nervous system even less than theophylline, but as the amount of theobromine in cacao products is seven or eight times higher than the caffeine concentration, the effect is still apparent. Like morphine and nicotine, caffeine (and theophylline and theobromine) are addictive compounds; withdrawal symptoms include headaches, fatigue, drowsiness, and evenâwhen caffeine intake has been excessiveânausea and vomiting. The good news is that caffeine clears the body relatively quickly, a week at mostâthough few of us have any intention of giving up on the world's favorite addiction.
Caffeine-containing plants were probably known to prehistoric man. They were almost certainly used in ancient times, but it is not possible to know whether tea, cacao, or coffee was the first. Legend has it that Shen Nung, the mythical first emperor of China, introduced the practice of boiling the drinking water for his court as a precaution against illness. One day he noticed that leaves from a nearby bush had fallen into the boiling water being prepared by his servants. The resulting infusion was supposedly the first of what must now be trillions of cups of tea that have been enjoyed in the five thousand years since. Although legends refer to drinking of tea in earlier times, Chinese literature does not mention tea, or its ability to “make one think better,” until the second century B.C. Other traditional Chinese stories indicate that tea may have been introduced from northern India or from Southeast Asia. Wherever the origin, tea has been a part of Chinese life for many centuries. In many Asian countries, particularly Japan, tea also became an important part of the national culture.
Other books
The Assassin Game by Kirsty McKay
Stevie Lee by Tara Janzen
White Dusk by Susan Edwards
Seduction by Molly Cochran
Polar City Blues by Katharine Kerr
HardWind by Charlotte Boyett-Compo
Girls on Film by Zoey Dean
The Payback Man by Carolyn McSparren
Danger Comes Home (Kelly O'Connell Mystery) by Alter, Judy
The nanny murders by Merry Bloch Jones