Penny le Couteur & Jay Burreson (41 page)
Read Penny le Couteur & Jay Burreson Online
Authors: Napoleon's Buttons: How 17 Molecules Changed History
Tags: #Philosophy & Social Aspects, #Science, #General, #World, #Chemistry, #Popular Works, #History

In medieval Europe the very same women who were persecuted kept alive the important knowledge of medicinal plants, as did native people in other parts of the world. Without these herbal traditions we might never have produced our present-day range of pharmaceuticals. But today, while we no longer execute those who value potent remedies from the plant world, we are eliminating the plants instead. The continuing loss of the world's tropical rain forests, now estimated at almost two million hectares each year, may deprive us of the discovery of other alkaloids that would be even more effective in treating a variety of conditions and diseases.
We may never know that there are molecules with antitumor properties, that are active against HIV, or that could be wonder drugs for schizophrenia, for Alzheimer's or Parkinson's disease in the tropical plants that are daily becoming closer to extinction. From a molecular point of view, the folklore of the past may be a key to our survival in the future.
13. MORPHINE, NICOTINE, AND CAFFEINE
G
IVEN THE HUMAN tendency to desire those things that make us feel good, it is not surprising that three different alkaloid moleculesâmorphine from the opium poppy, nicotine in tobacco, and caffeine in tea, coffee, and cocoaâhave been sought out and prized for millennia. But for every benefit these molecules have brought to mankind, they have also offered danger. Despite, or maybe because of, their addictive nature, they have affected many different societies in many ways. And all three came together unexpectedly at one intersection in history.
THE OPIUM WARSIVEN THE HUMAN tendency to desire those things that make us feel good, it is not surprising that three different alkaloid moleculesâmorphine from the opium poppy, nicotine in tobacco, and caffeine in tea, coffee, and cocoaâhave been sought out and prized for millennia. But for every benefit these molecules have brought to mankind, they have also offered danger. Despite, or maybe because of, their addictive nature, they have affected many different societies in many ways. And all three came together unexpectedly at one intersection in history.
Although it is nowadays mainly associated with the Golden Triangleâthe border region of the countries of Burma, Laos, and Thailandâthe opium poppy,
Papaver somniferum,
is native to the eastern Mediterranean region. The products of the opium poppy may have been gathered and appreciated since prehistoric times. Evidence suggests that more than five thousand years ago the properties of opium were known in the Euphrates River delta, generally credited as the site of the first recognizable human civilization. Archaeological indications of the use of opium at least three thousand years ago have been unearthed in Cyprus. Opium was included in the herbal lists and healing remedies of the Greeks, Phoenicians, Minoans, Egyptians, Babylonians, and other civilizations of antiquity. Supposedly around 330 B.C., Alexander the Great took opium to Persia and India, from where cultivation slowly spread eastward and reached China in about the seventh century.
Papaver somniferum,
is native to the eastern Mediterranean region. The products of the opium poppy may have been gathered and appreciated since prehistoric times. Evidence suggests that more than five thousand years ago the properties of opium were known in the Euphrates River delta, generally credited as the site of the first recognizable human civilization. Archaeological indications of the use of opium at least three thousand years ago have been unearthed in Cyprus. Opium was included in the herbal lists and healing remedies of the Greeks, Phoenicians, Minoans, Egyptians, Babylonians, and other civilizations of antiquity. Supposedly around 330 B.C., Alexander the Great took opium to Persia and India, from where cultivation slowly spread eastward and reached China in about the seventh century.
For hundreds of years opium remained a medical herb, either drunk as a bitter infusion or swallowed as a rolled pellet. By the eighteenth and particularly the nineteenth centuries artists, writers, and poets in Europe and the United States used opium to achieve a dreamlike state of mind that was thought to enhance creativity. Being less expensive than alcohol, opium also found a use by the poor as a cheap intoxicant. During these years its habit-forming qualities, if recognized, were seldom a concern. So pervasive was its use that even small babies and teething infants were dosed with opium preparations that were advertised as soothing syrups and cordials and that contained as much as 10 percent morphine. Laudanum, a solution of opium in alcohol often recommended for women, was widely consumed and available at any pharmacy without a prescription. It was a socially acceptable form of opium until it was prohibited in the early twentieth century.
In China opium had been a respected medicinal herb for hundreds of years. But the introduction of a new alkaloid-bearing plant, tobacco, changed the role of opium in Chinese society. Smoking was unknown in Europe until Christopher Columbus, at the end of his second voyage in 1496, brought tobacco back from the New World, where he had seen it in use. Tobacco use spread rapidly, despite severe penalties for its possession or importation in many Asian and Middle Eastern countries. In China in the middle of the seventeenth century the last emperor of the Ming dynasty prohibited the smoking of tobacco. Possibly the Chinese started to smoke opium as a substitute for the banned tobacco, as some reports suggest. Other historians credit the Portuguese from small trading posts on Formosa (now Taiwan) and Amoy in the East China Sea with introducing Chinese merchants to the idea of mixing opium with tobacco.
The effect of alkaloids such as morphine and nicotine, absorbed directly into the bloodstream through smoke inhaled into the lungs, is extraordinarily rapid and intense. When taken in this manner, opium quickly becomes addictive. By the beginning of the eighteenth century the smoking of opium was widespread throughout China. In 1729 an imperial edict banned the importation and sale of opium in China, but it was probably too late. An opium-smoking culture and a vast opium-related network of distribution and marketing already existed.
This is where our third alkaloid, caffeine, enters the story. Traders from Europe had previously found little satisfaction in trading with China. There were few commodities that China was willing to buy from the West, least of all the manufactured goods that the Dutch, British, French, and other European trading nations wanted to sell. But Chinese exports were in demand in Europe, particularly tea. Probably caffeine, the mildly addictive alkaloid molecule in tea, fueled the insatiable appetite of the West for the dried leaves of a shrub that had been grown since antiquity in China.
The Chinese were quite prepared to sell their tea, but they wanted to be paid in silver coin or bullion. For the British, buying tea with valuable silver was not their definition of trade. It soon became apparent that there was one commodity, though illegal, that the Chinese wanted and did not have. Thus Britain entered the opium business. Opium, cultivated in Bengal and other parts of British India by agents of the British East India Company, was sold to independent traders. It was then resold to Chinese importers, often under the protection of bribed Chinese officials. In 1839 the Chinese government attempted to halt this outlawed but flourishing trade. It confiscated and destroyed a year's supply of opium located in warehouses in Canton (present-day Guangzhou) and in British ships awaiting unloading in Canton's harbor. Only days later a group of drunken British sailors was accused of killing a local farmer, giving the British an excuse to declare war on China. British victory in what is now called the First Opium War (1839-1842) changed the balance of trade between the nations. China was required to pay a very large amount in reparations, to open five Chinese ports to British trade, and to cede Hong Kong as a British crown colony.
Nearly twenty years later another Chinese defeat in the Second Opium War, involving the French as well as the British, wrung further concessions from China. More ports were opened to foreign trade, Europeans were allowed the right of residence and travel, freedom of movement was given to Christian missionaries, and ultimately the opium trade was legalized. Opium, tobacco, and tea became responsible for breaking down centuries of Chinese isolation. China entered a period of upheaval and change that culminated in the Revolution of 1911.
IN THE ARMS OF MORPHEUSOpium contains twenty-four different alkaloids. The most abundant one, morphine, makes up about 10 percent of crude opium extract, a sticky, dried secretion from the poppy flowerpod. Pure morphine was first isolated from this poppy latex in 1803 by a German apothecary, Friedrich Serturner. He named the compound that he obtained morphine, after Morpheus, the Roman god of dreams. Morphine is a narcotic, a molecule that numbs the senses (thus removing pain) and induces sleep.
Intense chemical investigation followed Serturner's discovery, but the chemical structure of morphine was not finally determined until 1925. This 122-year delay should not be seen as unproductive. On the contrary, organic chemists often view the actual deciphering of the structure of morphine as equally beneficial to mankind as the well-known pain-relieving effects of this molecule. Classical methods of structure determination, new laboratory procedures, an understanding of the three-dimensional nature of carbon compounds, and new synthetic techniques were just some of the results of the unraveling of this marathon chemical puzzle. Structures of other important compounds have been deduced because of the work done on the composition of morphine.
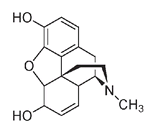
The structure of morphine. The darker lines of the wedge-shaped bonds point out of (above) the plane of the paper.
Today morphine and its related compounds are still the most effective painkillers known. Unfortunately, the painkilling or analgesic effect seems to be correlated with addiction. Codeine, a similar compound found in much smaller quantities (about 0.3 to 2 percent) in opium, is less addictive but is also a less powerful analgesic. The difference in structure is very small; codeine has a CH
3
O that replaces the HO at the position shown by the arrow on the structure below.
3
O that replaces the HO at the position shown by the arrow on the structure below.
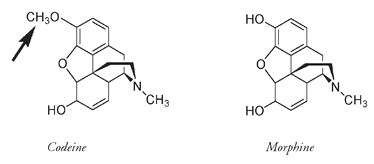
The structure of codeine. The arrow points to the only difference between codeine and morphine.
Well before the complete structure of morphine was known, attempts were made to modify it chemically in the hope of producing a compound that was a better pain reliever without addictive properties. In 1898, at the laboratory of Bayer and Company, the German dye manufacturer where, five years before, Felix Hofmann had treated his father with acetyl salicylic acid, chemists subjected morphine to the same acylation reaction that had converted salicylic acid to aspirin. Their reasoning was logical. Aspirin had turned out to be an excellent analgesic and a lot less toxic than salicylic acid.
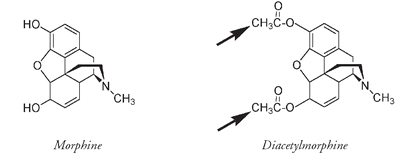
The diacetyl derivative of morphine. The arrows indicate where CH
3
CO has replaced the Hs in the two HOs of morphine, producing heroin.
3
CO has replaced the Hs in the two HOs of morphine, producing heroin.
The product of replacing the Hs of the two OH groups of morphine with CH
3
CO groups was, however, a different matter. At first the results seemed promising. Diacetylmorphine was an even more powerful narcotic than morphine, so effective that extremely low doses could be given. But its effectiveness masked a major problem, obvious when the commonly accepted name for diacetylmorphine is known. Originally marketed as Heroinâthe name refers to a “hero” drugâit is one of the most powerfully addictive substances known. The physiological effects of morphine and heroin are the same; inside the brain the diacetyl groups of heroin are converted back to the original OH groups of morphine. But the heroin molecule is more easily transported across the blood-brain barrier than is morphine, producing the rapid and intense euphoria craved by those who become addicted.
3
CO groups was, however, a different matter. At first the results seemed promising. Diacetylmorphine was an even more powerful narcotic than morphine, so effective that extremely low doses could be given. But its effectiveness masked a major problem, obvious when the commonly accepted name for diacetylmorphine is known. Originally marketed as Heroinâthe name refers to a “hero” drugâit is one of the most powerfully addictive substances known. The physiological effects of morphine and heroin are the same; inside the brain the diacetyl groups of heroin are converted back to the original OH groups of morphine. But the heroin molecule is more easily transported across the blood-brain barrier than is morphine, producing the rapid and intense euphoria craved by those who become addicted.
Bayer's Heroin, initially thought to be free of the common side effects of morphine like nausea and constipation and therefore assumed also to be free of addiction, was marketed as a cough suppressant and a remedy for headaches, asthma, emphysema, and even tuberculosis. But as the side effects of their “super aspirin” became obvious, Bayer and Company quietly stopped advertising it. When the original patents for acetyl salicylic acid expired in 1917 and other companies began producing aspirin, Bayer sued for breach of copyright over the name. Not surprisingly, Bayer has never sued for copyright violation of the Heroin trade name for diacetylmorphine.
Other books
The Best of Michael Swanwick by Swanwick, Michael
Witchy Sour (The Magic & Mixology Mystery Series Book 2) by Gina LaManna
When Only Love Remains by Durjoy Datta
Between Two Worlds by Zainab Salbi
Becoming a Jett Girl (The Bourbon Series) by Meghan Quinn
Tiberius by Allan Massie
PathFinder by Angie Sage
Trying to Float by Nicolaia Rips
Crazybone by Bill Pronzini
The Best Mistake by Kate Watterson