Penny le Couteur & Jay Burreson (17 page)
Read Penny le Couteur & Jay Burreson Online
Authors: Napoleon's Buttons: How 17 Molecules Changed History
Tags: #Philosophy & Social Aspects, #Science, #General, #World, #Chemistry, #Popular Works, #History

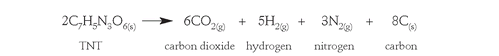
Carbon produced in this reaction causes the large amount of smoke that is associated with the explosions of TNT compared to those of nitroglycerin and guncotton.
At the beginning of World War I, Germany, using TNT-based munitions, had a definite advantage over the French and British, who were still using picric acid. A crash program to start producing TNT, aided by large quantities shipped from manufacturing plants in the United States, allowed Britain to rapidly develop similar quality shells and bombs containing this pivotal molecule.
Another molecule, ammonia (NH
3
), became even more crucial during World War I. While not a nitro compound, ammonia is the starting material for making the nitric acid, HNO
3
, which is needed to make explosives. Nitric acid has probably been known for a long time. Jabir ibn Hayyan, the great Islamic alchemist who lived around A.D. 800, would have known about nitric acid and probably made it by heating saltpeter (potassium nitrate) with ferrous sulfate (then called green vitriol because of its green crystals). The gas produced by this reaction, nitrogen dioxide (NO
2
), was bubbled into water to form a dilute solution of nitric acid.
3
), became even more crucial during World War I. While not a nitro compound, ammonia is the starting material for making the nitric acid, HNO
3
, which is needed to make explosives. Nitric acid has probably been known for a long time. Jabir ibn Hayyan, the great Islamic alchemist who lived around A.D. 800, would have known about nitric acid and probably made it by heating saltpeter (potassium nitrate) with ferrous sulfate (then called green vitriol because of its green crystals). The gas produced by this reaction, nitrogen dioxide (NO
2
), was bubbled into water to form a dilute solution of nitric acid.
Nitrates are not commonly found in nature, as they are very soluble in water and tend to be dissolved away, but in the extremely arid deserts of northern Chile huge deposits of sodium nitrate (so-called Chile saltpeter) have been mined for the past two centuries as a source of nitrate for direct preparation of nitric acid. Sodium nitrate is heated with sulfuric acid. The nitric acid that is produced is driven off because it has a lower boiling point than sulfuric acid. It is then condensed and collected in cooling vessels.
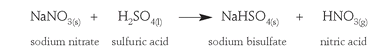
During World War I supplies of Chile saltpeter to Germany were cut off by a British naval blockade. Nitrates were strategic chemicals, necessary for manufacture of explosives, so Germany needed to find another source.
While nitrates may not be plentiful, the two elements, nitrogen and oxygen, that make up nitrates exist in the world in a generous supply. Our atmosphere is composed of approximately 20 percent oxygen gas and 80 percent nitrogen gas. Oxygen (O
2
) is chemically reactive, combining readily with many other elements, but the nitrogen molecule (N
2
) is relatively inert. At the beginning of the twentieth century, methods of “fixing” nitrogenâthat is, removing it from the atmosphere by chemical combination with other elementsâwere known but not very advanced.
2
) is chemically reactive, combining readily with many other elements, but the nitrogen molecule (N
2
) is relatively inert. At the beginning of the twentieth century, methods of “fixing” nitrogenâthat is, removing it from the atmosphere by chemical combination with other elementsâwere known but not very advanced.
For some time Fritz Haber, a German chemist, had been working on a process to combine nitrogen from the air with hydrogen gas to form ammonia.

Haber was able to solve the problem of using inert atmospheric nitrogen by working with reaction conditions that produced the highest yield of ammonia for the lowest possible cost: high pressure, temperatures of around 400 to 500°C, and removal of the ammonia as soon as it formed. Much of Haber's work involved finding a catalyst to increase the rate of this particularly slow reaction. His experiments were aimed at producing ammonia for the fertilizer industry. Two-thirds of the world's fertilizer needs were at that time being supplied from the saltpeter deposits in Chile; as these deposits became depleted, a synthetic route to ammonia was needed. By 1913 the world's first synthetic ammonia plant had been established in Germany, and when the British blockade later cut nitrate supply from Chile, the Haber process, as it is still known, was quickly expanded to other plants to supply ammonia not only for fertilizers but also for ammunition and explosives. The ammonia thus produced is reacted with oxygen to form nitrogen dioxide, the precursor of nitric acid. For Germany, with ammonia for fertilizers and nitric acid to make explosive nitro compounds, the British blockade was irrelevant. Nitrogen fixation had become a vital factor in waging war.
The 1918 Nobel Prize for chemistry was awarded to Fritz Haber for his role in the synthesis of ammonia, which ultimately led to increased fertilizer production and the consequent greater ability of agriculture to feed the world's population. The announcement of this award aroused a storm of protest because of the role Fritz Haber had played in Germany's gas warfare program in World War I. In April 1915 cylinders of chlorine gas had been released over a three-mile front near Ypres, Belgium. Five thousand men had been killed and another ten thousand suffered devastating effects on their lungs from chlorine exposure. Under Haber's leadership of the gas warfare program, a number of new substances, including mustard gas and phosgene, were also tested and used. Ultimately gas warfare was not the deciding factor in the outcome of the war, but in the eyes of many of his peers Haber's earlier great innovationâso crucial to world agricultureâdid not compensate for the appalling result of the exposure of thousands to poisonous gases. Many scientists considered awarding the Nobel Prize to Haber under these circumstances to be a travesty.
Haber saw little difference between conventional and gas warfare and was greatly upset by the controversy. In 1933, as director of the prestigious Kaiser Wilhelm Institute for Physical Chemistry and Electrochemistry, he was ordered by the Nazi government of Germany to dismiss all Jewish workers on his staff. In an unusual act of courage for those times, Haber refused, citing in his letter of resignation that “for more than forty years I have selected my collaborators on the basis of their intelligence and their character and not on the basis of their grand-mothers, and I am not willing for the rest of my life to change this method that I have found so good.”
Today, worldwide annual production of ammonia, still made by Haber's process, is about 140 million tons, much of it used for ammonium nitrate (NH
4
NO
3
), probably the world's most important fertilizer. Ammonium nitrate is also used for blasting in mines, as a mixture of 95 percent ammonium nitrate and 5 percent fuel oil. The explosive reaction produces oxygen gas as well as nitrogen and steam. The oxygen gas oxidizes the fuel oil in the mixture, increasing the energy released by the blast.
4
NO
3
), probably the world's most important fertilizer. Ammonium nitrate is also used for blasting in mines, as a mixture of 95 percent ammonium nitrate and 5 percent fuel oil. The explosive reaction produces oxygen gas as well as nitrogen and steam. The oxygen gas oxidizes the fuel oil in the mixture, increasing the energy released by the blast.
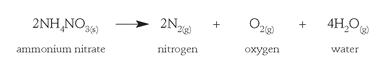
Ammonium nitrate is considered a very safe explosive when properly handled, but it has been responsible for a number of disasters as a result of improper safety procedures or deliberate bombings by terrorist organizations. In 1947, in the port of Texas City, Texas, a fire broke out in the hold of a ship as it was being loaded with paper bags of ammonium nitrate fertilizer. In an attempt to stop the fire, the ship's crew closed the hatches, which had the unfortunate effect of creating the conditions of heat and compression needed to detonate ammonium nitrate. More than five hundred people were killed in the ensuing explosion. More recent disasters involving ammonium nitrate bombs planted by terrorists include the incidents at the World Trade Center in New York City in 1993 and at the Alfred P. Murrah Federal Building in Oklahoma City in 1995.
One of the more recently developed explosives, pentaerythritoltetranitrate (abbreviated to PETN), is regrettably also favored by terrorists because of the very same properties that have made it so useful for legitimate purposes. PETN can be mixed with rubber to make what is called a plastic explosive, which can be pressed into any shape. PETN may have a complicated chemical name, but its structure is not that complicated. It is chemically similar to nitroglycerin but has five carbons instead of three and one more nitro group.
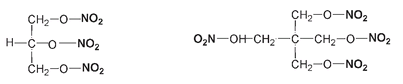
Nitroglycerin (left) and pentaerythritoltetranitrate (PETN) (right). The nitro groups are bolded.
Easily detonated, shock sensitive, very powerful, and with little odor so that even trained dogs find it difficult to detect, PETN may have become the explosive of choice for airplane bombings. It gained fame as a component of the bomb that brought down Pan Am flight 103 over Lockerbie, Scotland, in 1988. Further notoriety has resulted from the 2001 “Shoebomber” incident, in which a passenger on an American Airlines flight from Paris attempted to set off PETN hidden in the soles of his sneakers. Disaster was averted only due to quick action by crew and passengers.
Â
Â
The role of explosive nitro molecules has not been confined to wars and terrorism. There is evidence that the power of the saltpeter, sulfur, and charcoal mixture was used in mining in northern Europe by the early 1600s. The Malpas Tunnel (1679) of the Canal du Midi in France, the original canal linking the Atlantic Ocean to the Mediterranean Sea, was just the first of many major canal tunnels built with the help of gunpowder. The 1857-1871 building of the Mont Cenis or Fréjus railway tunnel, through the French Alps, was the largest use of explosive molecules of the time, changing the face of travel in Europe by allowing easy passage from France to Italy. The new explosive nitroglycerin was first used in construction in the Hoosac railway tunnel (1855-1866) at North Adams in Massachusetts. Major engineering feats have been accomplished with the aid of dynamite: the 1885 completion of the Canadian Pacific Railway, allowing passage through the Canadian Rockies; the eighty-kilometer-long Panama Canal, which opened in 1914; and the 1958 removal of the navigational hazard Ripple Rock off the west coast of North Americaâstill the largest-ever nonnuclear explosion.
Other books
Viper by Patricia A. Rasey
The Great Depression in United States History by David K. Fremon
Back To Me by Unknown
Devil's Plaything (Playthings, #1) by Lydia Rowan
Never Cry Wolf by Farley Mowat
Unavoidable Chance by Annalisa Nicole
Royal Hearts by Ruth Ann Nordin
Northwest Angle by William Kent Krueger
First to Dance by Writes, Sonya
The Journal of Vincent Du Maurier Trilogy (Books 1, 2, 3) by K. P. Ambroziak