Penny le Couteur & Jay Burreson (33 page)
Read Penny le Couteur & Jay Burreson Online
Authors: Napoleon's Buttons: How 17 Molecules Changed History
Tags: #Philosophy & Social Aspects, #Science, #General, #World, #Chemistry, #Popular Works, #History

Although Fleming published his results in a medical journal, they aroused little interest. His penicillin broth was very dilute, and his attempts to isolate the active ingredient were not successful; we now know that penicillin is easily inactivated by many common laboratory chemicals, and by solvents and heat.
Penicillin did not undergo clinical trials for more than a decade, during which time sulfanilamides became the major weapon against bacterial infections. In 1939 the success of sulfa drugs encouraged a group of chemists, microbiologists, and physicians at Oxford University to start working on a method to produce and isolate penicillin. The first clinical trial with crude penicillin was in 1941. Sadly, the results were much like the old punch line “The treatment was a success, but the patient died.” Intravenous penicillin treatment was given to one patient, a policeman suffering from both severe staphylococcal and streptococcal infections. After twenty-four hours an improvement was noted; five days later his fever was gone and his infection was clearing. But by then all of the penicillin availableâabout a teaspoon of the unrefined extractâhad been used up. The man's infection was still virulent. It expanded unchecked, and he soon died. A second patient also died. In a third trial, however, enough penicillin had been produced to completely eliminate a streptococcal infection in a fifteen-year-old boy. After that success penicillin cured staphylococcal blood poisoning in another child, and the Oxford group knew they had a winner. Penicillin proved active against a range of bacteria, and it had no harsh side effects, such as the kidney toxicity that had been reported with sulfanilamides. Later studies indicated that some penicillins inhibit the growth of streptococci at a dilution of one to fifty million, an amazingly small concentration.
At this time the chemical structure of penicillin was not yet known, and so it was not possible to make it synthetically. Penicillin still had to be extracted from molds, and the production of large amounts was a challenge for microbiologists and bacteriologists rather than chemists. The U.S. Department of Agriculture laboratory in Peoria, Illinois, had expertise in growing microorganisms and became the center of a massive research program. By July 1943 American pharmaceutical companies were producing 800 million units of the new antibiotic. One year later the monthly production topped 130 billion units.
It has been estimated that during World War II a thousand chemists in thirty-nine laboratories in the United States and in Britain worked on the problems associated with establishing the chemical structure of and finding a way to synthesize penicillin. Finally, in 1946, the structure was determined, although it was successfully synthesized only in 1957.
The structure of penicillin may not be as large or look as complicated a molecule as others we have discussed, but for chemists it is a most unusual molecule in that it contains a four-membered ring, known in this case as the β-lactam ring.
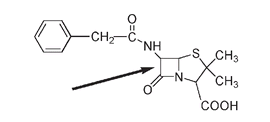
The structure of the penicillin G molecule. The arrow indicates the four-membered
β
-lactam ring.
β
-lactam ring.
Molecules with four-membered rings do exist in nature, but they are not common. Chemists can make such compounds, but it can be quite difficult. The reason is that the angles in a four-membered ringâa squareâare 90 degrees, while normally the preferred bond angles for single-bonded carbon and nitrogen atoms are near 109 degrees. For a double-bonded carbon atom, the preferred bond angle is around 120 degrees.
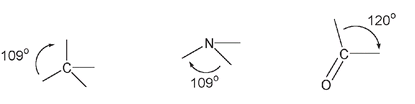
The single-bonded carbon and nitrogen atoms are three-dimensionally arranged in space, while the carbon double-bonded to an oxygen atom is in the same plane.
In organic compounds a four-membered ring is not flat; it buckles slightly, but even this cannot reduce what chemists call
ring strain,
an instability that results mainly from atoms being forced to have bond angles too different from the preferred bond angle. But it is precisely this instability of the four-membered ring that accounts for the antibiotic activity of penicillin molecules. Bacteria have cell walls and produce an enzyme that is essential for cell wall formation. In the presence of this enzyme, the β-lactam ring of the penicillin molecule splits open, relieving ring strain. In the process an OH group on the bacterial enzyme is
acylated
(the same type of reaction that converted salicylic acid into aspirin). In this acylation reaction penicillin attaches the ring-opened molecule to the bacterial enzyme. Note that the five-membered ring is still intact, but the four-membered ring has opened up.
ring strain,
an instability that results mainly from atoms being forced to have bond angles too different from the preferred bond angle. But it is precisely this instability of the four-membered ring that accounts for the antibiotic activity of penicillin molecules. Bacteria have cell walls and produce an enzyme that is essential for cell wall formation. In the presence of this enzyme, the β-lactam ring of the penicillin molecule splits open, relieving ring strain. In the process an OH group on the bacterial enzyme is
acylated
(the same type of reaction that converted salicylic acid into aspirin). In this acylation reaction penicillin attaches the ring-opened molecule to the bacterial enzyme. Note that the five-membered ring is still intact, but the four-membered ring has opened up.
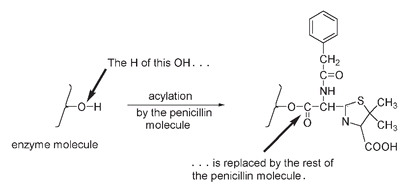
The penicillin molecule attaches to the bacterial enzyme in this acylation reaction.
This acylation deactivates the cell wall-forming enzyme. Without the ability to build cell walls, the growth of new bacteria in an organism is inhibited. Animal cells have a cell membrane rather than a cell wall and so do not have the same wall-forming enzyme as these bacteria. We are therefore not affected by the acylation reaction with the penicillin molecule.
The instability of the four-membered β-lactam ring of penicillin is also the reason that penicillins, unlike sulfa drugs, need to be stored at low temperatures. Once the ring opensâa process accelerated by heatâthe molecule is no longer an effective antibiotic. Bacteria themselves seem to have discovered the secret of ring opening. Penicillin-resistant strains have developed a further enzyme that breaks open the β-lactam ring of penicillin before it has a chance to deactivate the enzyme responsible for cell wall formation.
The structure of the penicillin molecule shown below is that of penicillin G, first produced from mold in 1940 and still widely used. Many other penicillin molecules have been isolated from molds, and a number have been synthesized chemically from the naturally occurring versions of this antibiotic. The structures of different penicillins vary only in the part of the molecule circled below.
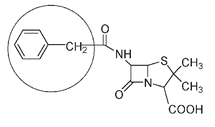
Penicillin G. The variable part of the molecule is circled.
Ampicillin, a synthetic penicillin effective against bacteria that are resistant to penicillin G, is only slightly different. It has an extra NH
2
group attached.
2
group attached.
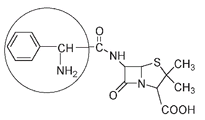
Ampicillin
The side group in amoxicillin, today one of the most widely prescribed drugs in the United States, is very similar to ampicillin but with an extra OH. The side group can be very simple, as in penicillin O, or more complicated, as in cloxacillin.
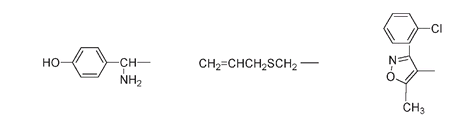
The structure of the side groups in the circled portion of the molecule for amoxicillin (left), penicillin O (center), and cloxacillin (right)
These are only four of the ten or so different penicillins still in use today. (Many more exist that are no longer used clinically.) The structural modifications, at the same (circled) site on the molecule, can be very variable, but the four-membered β-lactam ring is always present. It is this piece of the molecular structure that may have saved your life, if you have had the occasion to need a penicillin antibiotic.
Â
Â
Although it is impossible to obtain accurate statistics of mortality during previous centuries, demographers have estimated average lifespans in some societies. From 3500 B.C. through to about A.D. 1750, a period of over five thousand years, life expectancy among European societies hovered between thirty and forty years; in classical Greece, around 680 B.C., it rose as high as forty-one years; in Turkey of A.D. 1400 it was just thirty-one years. These numbers are similar to those in the underdeveloped countries of the world today. The three main reasons for these high mortality ratesâinadequate food supplies, poor sanitation, and epidemic diseaseâare closely interrelated. Poor nutrition leads to increased susceptibility to infection; poor sanitation produces conditions conducive to disease.
In those parts of the world with efficient agriculture and a good transportation system, food supply has increased. At the same time vastly improved personal hygiene and public health measuresâclean water supply, sewage treatment systems, refuse collection and vermin control, and wholesale immunization and vaccination programsâhave led to fewer epidemics and a healthier population more able to resist disease. Because of these improvements, death rates in the developed world have been dropping steadily since the 1860s. But the final onslaught against those bacteria that have for generations caused untold misery and death has been by antibiotics.
Other books
The Knight Of The Rose by A. M. Hudson
Borderlines by Archer Mayor
Cheri on Top by Susan Donovan
Taken By The Pack (Werewolf's Harem Book 2) by Vivian Wood
The Other Ida by Amy Mason
T Wave by Steven F. Freeman
The Bad Girls' Club by O'Halloran, Kathryn
Amok and Other Stories by Stefan Zweig
Testimony Of Two Men by Caldwell, Taylor
Blue Damask by Banks, Annmarie