Penny le Couteur & Jay Burreson (31 page)
Read Penny le Couteur & Jay Burreson Online
Authors: Napoleon's Buttons: How 17 Molecules Changed History
Tags: #Philosophy & Social Aspects, #Science, #General, #World, #Chemistry, #Popular Works, #History

ASPIRIN
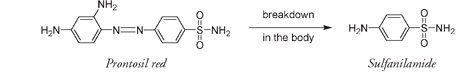
In the early twentieth century the German and Swiss chemical industries were prospering from their investment in the manufacture of dyestuffs. But this success was more than just financial. Along with profits from dye sales came a new wealth of chemical knowledge, of experience with large-scale reactions, and of techniques for separation and purification that were vital for expansion into the new chemical business of pharmaceuticals. Bayer and Company, the German firm that got its start from aniline dyes, was one of the first to recognize the commercial possibilities in the chemical production of medicinesâin particular aspirin, which has now been used by more people worldwide than any other medication.
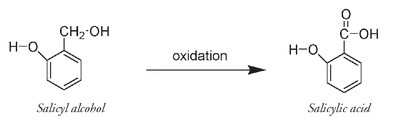
In 1893 Felix Hofmann, a chemist working for the Bayer company, decided to investigate the properties of compounds that were related to salicylic acid, a molecule obtained from salicin, a pain-relieving molecule originally isolated from the bark of trees of the willow genus (
Salix
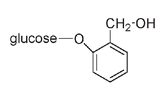
) in 1827. The curative properties of the willow and related plants such as poplars had been known for centuries. Hippocrates, the famed physician of ancient Greece, had used extracts from willow bark to reduce fevers and relieve pain. Although the bitter-tasting salicin molecule incorporates a glucose ring into its structure, the rest of the molecule overwhelms any sweetness from the sugar part.
Salix
) in 1827. The curative properties of the willow and related plants such as poplars had been known for centuries. Hippocrates, the famed physician of ancient Greece, had used extracts from willow bark to reduce fevers and relieve pain. Although the bitter-tasting salicin molecule incorporates a glucose ring into its structure, the rest of the molecule overwhelms any sweetness from the sugar part.

The salicin molecule
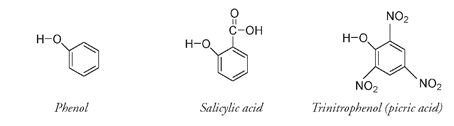
Like the glucose-containing indican molecule that produces indigo, salicin breaks into two parts: glucose and salicyl alcohol, which can be oxidized to salicylic acid. Both salicyl alcohol and salicylic acid are classified as phenols because they have an OH group directly attached to the benzene ring.
These molecules are also similar in structure to isoeugenol, eugenol, and zingerone from cloves, nutmeg, and ginger. It is probable that like these molecules, salicin acts as a natural pesticide to protect the willow tree. Salicylic acid is also produced from the flowers of meadowsweet or
Spiraea ulmaria,
a wetlands perennial native to Europe and western Asia.
Spiraea ulmaria,
a wetlands perennial native to Europe and western Asia.
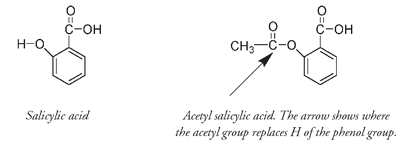
Salicylic acid, the active portion of the salicin molecule, not only reduces fever and relieves pain but also acts as an anti-inflammatory. It is much more potent than the naturally occurring salicin, but it can be very irritating to the lining of the stomach, reducing its medicinal value. Hofmann's interest in compounds related to salicylic acid arose out of concern for his father, whose rheumatoid arthritis was little relieved by salicin. Hoping that the anti-inflammatory properties of salicylic acid would be retained but its corrosive properties lessened, Hofmann gave his father a derivative of salicylic acidâacetyl salicylic acid, first prepared by another German chemist forty years previously. In ASA, as acetyl salicylic acid has come to be called, the acetyl group (CH
3
CO) replaces the H of the phenolic OH group of salicylic acid. The phenol molecule is corrosive; perhaps Hofmann reasoned that converting the OH attached to the aromatic ring into an acetyl group might mask its irritating characteristics.
3
CO) replaces the H of the phenolic OH group of salicylic acid. The phenol molecule is corrosive; perhaps Hofmann reasoned that converting the OH attached to the aromatic ring into an acetyl group might mask its irritating characteristics.

Hofmann's experiment paid offâfor his father and for the Bayer company. The acetylated form of salicylic acid turned out to be effective and well tolerated. Its potent anti-inflammatory and analgesic properties persuaded the Bayer company, in 1899, to begin marketing small packets of powdered “aspirin.” The name is a combination of the
a
from
acetyl
and the
spir
from
Spiraea ulmaria,
the meadowsweet plant. The Bayer company name became synonymous with aspirin, marking Bayer's entrance into the world of medicinal chemistry.
a
from
acetyl
and the
spir
from
Spiraea ulmaria,
the meadowsweet plant. The Bayer company name became synonymous with aspirin, marking Bayer's entrance into the world of medicinal chemistry.
As the popularity of aspirin increased, the natural sources from which salicylic acid was producedâmeadowsweet and willowâwere no longer sufficient to satisfy world demand. A new synthetic method using the phenol molecule as the starting material was introduced. Aspirin sales soared; during World War I the American subsidiary of the original Bayer company purchased as much phenol as possible from both national and international sources in order to guarantee an adequate supply for the manufacture of aspirin. The countries that supplied Bayer with phenol thus had reduced capacity to make picric acid (trinitrophenol), an explosive also prepared from this same starting material (see Chapter 5). What effect this may have had on the course of World War I we can only speculate, but aspirin production may have reduced reliance on picric acid for munitions and hastened the development of TNT-based explosives.

Today aspirin is the most widely used of all drugs for treating illness and injury. There are well over four hundred aspirin-containing preparations, and over forty million pounds of aspirin are produced in the United States annually. As well as relieving pain, lowering body temperature, and reducing inflammation, aspirin also has blood-thinning properties. Small doses of aspirin are being recommended as a preventive against strokes and for deep-vein thrombosis, the condition known as “economy class syndrome” in long-haul airline passengers.
THE SAGA OF SULFAAround the time of Hofmann's experiment on his fatherâa drug-trial procedure that is not recommendedâthe German doctor Paul Ehrlich was conducting experiments of his own. Ehrlich was, by all accounts, a truly eccentric character, said to smoke twenty-five cigars a day and spend many hours in philosophical discussions in beer halls. But along with his eccentricity came the determination and insight that gained him the 1908 Nobel Prize in medicine. Despite having no formal training in experimental chemistry or applied bacteriology, Ehrlich noted that different coal tar dyes would stain some tissues and some microorganisms but not others. He reasoned that if one microorganism absorbed a dyestuff and another did not, this differentiation might allow a toxic dye to kill tissue that absorbed it without damaging nonstaining tissue. Hopefully the infecting microorganism would be eliminated while the host was unharmed. Ehrlich termed this theory the “magic bullet” approach, the magic bullet being the dye molecule targeting the tissue it stained.
Ehrlich's first success was with a dye called trypan red I, which acted very much as he had hoped against trypanosomesâa protozoic parasiteâin laboratory mice. Unfortunately it was not effective against the type of trypanosome responsible for the human disease known as African sleeping sickness, which Ehrlich had hoped to cure.
Undeterred, Ehrlich continued. He had shown that his method could work, and he knew it was only a matter of finding a suitable magic bullet for the right disease. He began investigating syphilis, an affliction caused by a corkscrew-shaped bacterium known as a spirochete. Theories of how syphilis came to Europe abound; one of the most widely acknowledged was that it returned from the New World with Columbus's sailors. A form of “leprosy” reported in Europe before Columbus's time, however, was known to be highly contagious and venereally spread. Like syphilis it also sometimes responded to treatment with mercury. None of these observations fit what we know about leprosy, and it is possible that what was described was actually syphilis.
By the time Ehrlich began looking for a magic bullet against this bacterium, mercury cures had been claimed for syphilis for over four hundred years. Yet mercury could hardly be considered a magic bullet for syphilis, as it often killed its patients. Victims died of heart failure, dehydration, and suffocation during the process of being heated in an oven while breathing mercury fumes. If one survived this procedure, typical symptoms of mercury poisoningâloss of hair and teeth, uncontrollable drooling, anemia, depression, and kidney and liver failureâtook their toll.
In 1909, after testing 605 different chemicals, Ehrlich finally found a compound that was both reasonably effective and reasonably safe. “Number 606,” an arsenic-containing aromatic compound, proved active against the syphilis spirochete. Hoechst Dyeworksâthe company Ehrlich collaborated withâmarketed this compound in 1910, under the name salvarsan. Compared with the torture of the mercury remedy, the new treatment was a great improvement. Despite some toxic side effects and the fact that it did not always cure syphilitic patients even after a number of treatments, salvarsan greatly reduced the incidence of the disease wherever it was used. For Hoechst Dyeworks it proved extremely profitable, providing the capital to diversify into other pharmaceuticals.
After the achievement of salvarsan, chemists sought further magic bullets by testing tens of thousands of compounds for their effect on microorganisms, then making slight changes to chemical structures and testing again. There were no successes. It seemed as if the promise of what Ehrlich had termed “chemotherapy” would not live up to expectations. But then in the early 1930s Gerhard Dogmak, a doctor working with the IG Farben research group, decided to use a dye called prontosil red to treat his daughter, who was desperately ill with a streptococcal infection contracted from a simple pinprick. He had been experimenting with prontosil red at the IG Farben laboratory, and though it had shown no activity against bacteria grown in laboratory cultures, it did inhibit the growth of streptococci in laboratory mice. No doubt deciding he had nothing to lose, Dogmak gave his daughter an oral dose of the still-experimental dye. Her recovery was fast and complete.
It was at first assumed that the dye actionâthe actual staining of the cellsâwas responsible for the antibacterial properties of prontosil red. But researchers soon realized that antibacterial effects had nothing to do with dye action. In the human body the prontosil red molecule breaks down to produce sulfanilamide, and it is the sulfanilamide that has the antibiotic effect.
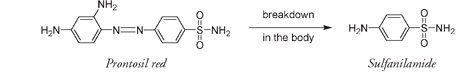
Other books
Fake (A Pretty Pill) by Criss Copp
A Mate Beyond Their Reach by Hyacinth, Scarlet
Here at the End of the World We Learn to Dance by Lloyd Jones
A Dreadful Past by Peter Turnbull
Courtney Milan by A Novella Collection
Forbidden Valentine: A Forbidden Novel by Valentine, J.C.
La Hermandad de la Sábana Santa by Julia Navarro
The Library of Greek Mythology (Oxford World's Classics) by Apollodorus, Robin Hard
Stuka Pilot by Hans-Ulrich Rudel
Hero Found: The Greatest POW Escape of the Vietnam War by Bruce Henderson