Penny le Couteur & Jay Burreson (34 page)
Read Penny le Couteur & Jay Burreson Online
Authors: Napoleon's Buttons: How 17 Molecules Changed History
Tags: #Philosophy & Social Aspects, #Science, #General, #World, #Chemistry, #Popular Works, #History

From the 1930s the effect of these molecules on mortality rates from infectious diseases has been marked. After the introduction of sulfa drugs to treat pneumonia, a common complication with the measles virus, the death rate from measles declined rapidly. Pneumonia, tuberculosis, gastritis, and diphtheria, all among leading causes of death in the United States in 1900, do not make the list today. Where isolated incidents of bacterial diseasesâbubonic plague, cholera, typhus, and anthraxâhave occurred, antibiotics contained what might otherwise have become a widespread outbreak. Today's acts of bioterrorism have focused public concern on the possibility of a major bacterial epidemic. Our present array of antibiotics would normally be able to cope with such an attack.
Another form of bioterrorism, that waged by bacteria themselves as they adapt to our increasing use and even overuse of antibiotics, is worrying. Antibiotic-resistant strains of some common but potentially lethal bacteria are becoming widespread. But as biochemists learn more about the metabolic pathways of bacteriaâand of humansâand how the older antibiotics worked, it should become possible to synthesize new antibiotics able to target specific bacterial reactions. Understanding chemical structures and how they interact with living cells is essential to maintaining an edge in the never-ending struggle with disease-causing bacteria.
11. THE PILL
B
Y THE MIDDLE of the twentieth century antibiotics and antiseptics were in common use and had dramatically lowered mortality rates, particularly among women and children. Families no longer needed a multitude of children to ensure that some reached maturity. As the specter of losing children to infectious diseases diminished, a demand for ways of limiting family size by preventing conception arose. In 1960 a contraceptive molecule emerged that played a major role in shaping contemporary society.
Y THE MIDDLE of the twentieth century antibiotics and antiseptics were in common use and had dramatically lowered mortality rates, particularly among women and children. Families no longer needed a multitude of children to ensure that some reached maturity. As the specter of losing children to infectious diseases diminished, a demand for ways of limiting family size by preventing conception arose. In 1960 a contraceptive molecule emerged that played a major role in shaping contemporary society.
We are, of course, referring to
norethindrone,
the first oral contraceptive, usually known as “the pill.” The molecule has been credited withâor blamed for (depending on your point of view)âthe sexual revolution of the 1960s, the women's liberation movement, the rise of feminism, the increased percentage of women in the workplace, and even the breakdown of the family. Despite the varying opinions on the benefits or disadvantages of this molecule, it has played an important role in the enormous changes in society in the forty or so years since the pill was introduced.
norethindrone,
the first oral contraceptive, usually known as “the pill.” The molecule has been credited withâor blamed for (depending on your point of view)âthe sexual revolution of the 1960s, the women's liberation movement, the rise of feminism, the increased percentage of women in the workplace, and even the breakdown of the family. Despite the varying opinions on the benefits or disadvantages of this molecule, it has played an important role in the enormous changes in society in the forty or so years since the pill was introduced.
Struggles for legal access to birth control information and supplies, fought in the early part of the last century by such notable reformers as Margaret Sanger in the United States and Marie Stopes in Britain, seem remote to us now. Young people today are often incredulous on hearing that just to give information on contraception was, in many countries during the initial decades of the twentieth century, a crime. But the need was clearly present: high rates of infant mortality and maternal death found in poor areas of cities often correlated with large families. Middle-class families were already using the contraceptive methods then available, and working-class women were desperate for the same information and access. Letters written to birth control advocates by mothers of large families detailed the despair they felt while facing yet another unwanted pregnancy. By the 1930s public acceptance of birth control, often couched in the more acceptable term
family planning,
was increasing; health clinics and medical personnel were involved in prescribing contraceptive devices, and laws, at least in some places, were being changed. Where restrictive statutes did remain on the books, prosecutions became less common, especially if contraception matters were handled discreetly.
EARLY ATTEMPTS AT ORAL CONTRACEPTIONfamily planning,
was increasing; health clinics and medical personnel were involved in prescribing contraceptive devices, and laws, at least in some places, were being changed. Where restrictive statutes did remain on the books, prosecutions became less common, especially if contraception matters were handled discreetly.
Over the centuries and in every culture women have swallowed many substances in the hope of preventing conception. None of these substances would have accomplished this goal except, perhaps, by making the woman so ill she would be unable to conceive. Some of the remedies were fairly straightforward: brewed teas of parsley and mint, of leaves or bark of hawthorn, ivy, willow, wallflower, myrtle, or poplar. Mixtures containing spiders' eggs or snake were also suggested. Fruits, flowers, kidney beans, apricot kernels, and mixed herbal potions were other recommendations. At one time the mule featured prominently in contraception, supposedly because a mule is the sterile offspring of a female horse and a male donkey. Sterility was allegedly assured if a woman ate the kidney or uterus of a mule. For male sterility the animal's contribution was no less tasty; a man was to eat the burned testicles of a castrated mule. Mercury poisoning might have been an effective means of attaining sterility for a woman swallowing a seventh-century Chinese remedy of quicksilver (an old name for mercury) fried in oilâthat is, if it did not kill her first. Solutions of different copper salts were drunk as contraceptives in ancient Greece and in parts of Europe in the 1800s. A bizarre method from the Middle Ages required a woman to spit three times into the mouth of a frog. It was the woman who would become sterile, not the frog!
STEROIDSThough some of the substances smeared on various parts of the body to prevent pregnancy could possibly have had spermicidal properties, the advent of oral contraceptives in the middle of the twentieth century marked the first truly safe and effective chemical means of birth control. Norethindrone is one of a group of compounds known as
steroids,
a perfectly good chemical name that now is often applied to performance-enhancing drugs illegally used by some athletes. Such drugs are definitely steroids, but so are many other compounds that have nothing to do with athletic prowess; we will be using the term
steroid
in the wider chemical sense.
steroids,
a perfectly good chemical name that now is often applied to performance-enhancing drugs illegally used by some athletes. Such drugs are definitely steroids, but so are many other compounds that have nothing to do with athletic prowess; we will be using the term
steroid
in the wider chemical sense.
In many molecules very small changes in structure can have very large changes in effect. This is nowhere more pronounced than in the structures of the sex hormones: the male sex hormones (androgens), the female sex hormones (estrogens), and the hormones of pregnancy (progestins).
All compounds that are classified as steroids have the same basic molecular pattern, a series of four rings fused in the same way. Three of the rings have six carbon atoms each, and the fourth has five. These rings are referred to as the A, B, C, and D ringsâthe D ring always being five-membered.
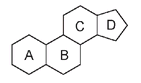
The four basic rings of the steroid structure, showing the A, B, C, and D designations
Cholesterol, the most widespread of all animal steroids, is found in most animal tissues, with especially high levels in egg yolks and human gall-stones. It is a molecule with an undeservedly bad reputation. We need cholesterol in our system; it plays a vital role as the precursor molecule of all our other steroids, including bile acids (compounds that enable us to digest fats and oils) and the sex hormones. What we do not need is a lot of extra cholesterol in our diet, as we synthesize enough of our own. The molecular structure of cholesterol shows the four basic fused rings as well as the side groups, including a number of methyl groups (CH
3
, sometimes written as H
3
C, just to fit more easily on the drawing).
3
, sometimes written as H
3
C, just to fit more easily on the drawing).
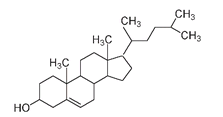
Cholesterol, the most widespread animal steroid
Testosterone, the principal male sex hormone, was first isolated from ground-up bull testes in 1935, but it was not the first male sex hormone to be isolated. That hormone was androsterone, a metabolized and less potent variation of testosterone that is excreted in the urine. As you can see from a comparison of the two structures, there is very little difference between them, androsterone being an oxidized version where a double-bonded oxygen atom has replaced the OH of testosterone.
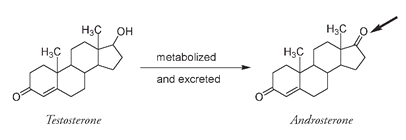
Androsterone varies from testosterone at only one position (arrowed).
The first isolation of a male hormone was in 1931 when fifteen milligrams of androsterone was obtained from fifteen thousand liters of urine collected from the Belgian police force, presumably an all-male group in those days.
The first sex hormone ever isolated was the female sex hormone estrone, obtained in 1929 from urine of pregnant women. As with androsterone and testosterone, estrone is a metabolized variation of the principal and more potent female sex hormone, estradiol. A similar oxidation process changes an OH on estradiol into a double-bonded oxygen.
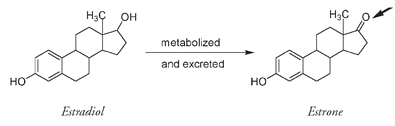
Estrone varies from estradiol at only one position (arrowed).
These molecules are present in our bodies in very small amounts: four tons of pig ovaries were used to extract only twelve milligrams of the first estradiol that was isolated.
It is interesting to consider how structurally similar the male hormone testosterone and the female hormone estradiol are. Just a few changes in molecular structure make an enormous difference.
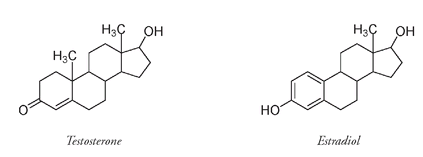
If you have one less CH
3
, an OH instead of a double-bonded O, and a few more C=C bonds, then at puberty instead of developing male secondary sex characteristics (facial and body hair, deep voice, heavier muscles), you will grow breasts and wider hips and start menstruating.
3
, an OH instead of a double-bonded O, and a few more C=C bonds, then at puberty instead of developing male secondary sex characteristics (facial and body hair, deep voice, heavier muscles), you will grow breasts and wider hips and start menstruating.
Other books
Charlotte Cuts It Out by Kelly Barson
Crow Lake by Mary Lawson
The Believers by Zoë Heller
Jam and Roses by Mary Gibson
On Common Ground (Harlequin Super Romance) by Kelleher, Tracy
The Dead Lie Down by Sophie Hannah
Blue Noon by Scott Westerfeld
Ice Fire: A Jock Boucher Thriller by David Lyons
Memory Boy by Will Weaver