Penny le Couteur & Jay Burreson (51 page)
Read Penny le Couteur & Jay Burreson Online
Authors: Napoleon's Buttons: How 17 Molecules Changed History
Tags: #Philosophy & Social Aspects, #Science, #General, #World, #Chemistry, #Popular Works, #History

A true refrigerator needs a refrigerantâa compound that undergoes the evaporation-compression cycle. As early as 1748 ether was used to demonstrate the cooling effect of a refrigerant, but it was more than a hundred years before a compressed ether machine was employed as a refrigerator. Around 1851, James Harrison, a Scotsman who had immigrated to Australia in 1837, built an ether-based vapor-compression refrigerator for an Australian brewery. He and an American, Alexander Twining, who had made a similar vapor-compression refrigeration system at about the same time, are considered to be among the first developers of commercial refrigeration.
Ammonia was used as a refrigerant in 1859, by Ferdinand Carré of Franceâanother claimant to the title of first commercial developer of refrigeration. Methyl chloride and sulfur dioxide were also used in these early days; sulfur dioxide was the cooling agent for the world's first artificial skating rink. These small molecules effectively ended the reliance on salt and spices for food preservation.

In 1873, after successfully establishing land-based refrigeration for the Australian meat-packing industry as well as the breweries, James Harrison decided to transport meat on a refrigerated ship from Australia to Britain. But his ether-based evaporation-compression mechanical system failed at sea. Then in early December 1879 the S.S.
Strathleven,
equipped by Harrison, left Melbourne and arrived in London two months later with forty tons of still-frozen beef and mutton. Harrison's refrigeration process was proven. In 1882 a similar system was installed on the S.S.
Dunedin,
and the first cargo of New Zealand lamb was shipped to Britain. Though the
Frigorifique
is often referred to as the world's first refrigerated ship, technically the claim better fits Harrison's 1873 attempt. It was not, however, the first
successful
voyage of a refrigerated ship. This latter title more rightly belongs to the S.S.
Paraguay,
which arrived in Le Havre, France, in 1877 with a cargo of frozen beef from Argentina. The
Paraguay
's refrigeration system was designed by Ferdinand Carré and used ammonia as a refrigerant.
Strathleven,
equipped by Harrison, left Melbourne and arrived in London two months later with forty tons of still-frozen beef and mutton. Harrison's refrigeration process was proven. In 1882 a similar system was installed on the S.S.
Dunedin,
and the first cargo of New Zealand lamb was shipped to Britain. Though the
Frigorifique
is often referred to as the world's first refrigerated ship, technically the claim better fits Harrison's 1873 attempt. It was not, however, the first
successful
voyage of a refrigerated ship. This latter title more rightly belongs to the S.S.
Paraguay,
which arrived in Le Havre, France, in 1877 with a cargo of frozen beef from Argentina. The
Paraguay
's refrigeration system was designed by Ferdinand Carré and used ammonia as a refrigerant.
On the
Frigorifique
the “refrigeration” was maintained by water that was cooled by ice (stored in a well-insulated room) and then pumped around the ship in pipes. The ship's pump broke down on the journey from Buenos Aires, and the meat was spoiled before it arrived in France. So although the
Frigorifique
predated the S.S.
Paraguay
by a number of months, it was not a true refrigerated ship; it was only an insulated ship, keeping food chilled or frozen with stored ice. What the
Frigorifique
can claim to be is a pioneer in transporting chilled meat across the ocean, even if it was not a successful pioneer.
Frigorifique
the “refrigeration” was maintained by water that was cooled by ice (stored in a well-insulated room) and then pumped around the ship in pipes. The ship's pump broke down on the journey from Buenos Aires, and the meat was spoiled before it arrived in France. So although the
Frigorifique
predated the S.S.
Paraguay
by a number of months, it was not a true refrigerated ship; it was only an insulated ship, keeping food chilled or frozen with stored ice. What the
Frigorifique
can claim to be is a pioneer in transporting chilled meat across the ocean, even if it was not a successful pioneer.
Irrespective of whose claim to the first refrigerated ship is most valid, by the 1880s the mechanical compression-evaporation process was set to solve the problem of transporting meat from the producing areas of the world to the larger markets of Europe and the eastern United States. Ships from Argentina and the even more distant cattle and sheep pastures of Australia and New Zealand faced a two- or three-month journey through the warm temperatures of the tropics. The simple ice system of the
Frigorifique
would not have been effective. Mechanical refrigeration began to increase in reliability, giving ranchers and farmers a new means of getting their products to world markets. Refrigeration thus played a major role in the economic development of Australia, New Zealand, Argentina, South Africa, and other countries, where great distances from markets reduced their natural advantages of abundant agricultural production.
FABULOUS FREONSFrigorifique
would not have been effective. Mechanical refrigeration began to increase in reliability, giving ranchers and farmers a new means of getting their products to world markets. Refrigeration thus played a major role in the economic development of Australia, New Zealand, Argentina, South Africa, and other countries, where great distances from markets reduced their natural advantages of abundant agricultural production.
The ideal refrigerant molecule has special practical requirements. It must vaporize within the right temperature range; it must liquefy by compressionâagain within the required temperature range; and it must absorb relatively large amounts of heat as it vaporizes. Ammonia, ether, methyl chloride, sulfur dioxide, and similar molecules satisfied these technical requirements as good refrigerants. But they either decomposed, were fire hazards, were poisonous, or smelled terribleâsometimes all of these.
Despite the problems with refrigerants, the demand for refrigeration, both commercial and domestic, grew. Commercial refrigeration, developed to meet the demand of trade, preceded home refrigeration by fifty or more years. The first refrigerators for in-home use became available in 1913 and by the 1920s had begun to replace the more traditional icebox, supplied with ice from industrial ice plants. In some early home refrigerators the noisy compressor unit was installed in the basement, separate from the food box.
Looking for an answer to concerns about toxic and explosive refrigerants, mechanical engineer Thomas Midgley, Jr.âalready successful as the developer of tetraethyl lead, a substance added to gasoline to reduce engine knockâand chemist Albert Henne, working at the Frigidaire Division of General Motors, considered compounds that were likely to have boiling points within the defined range of a refrigeration cycle. Most of the known compounds that fitted this criterion were already in use or had been eliminated as impractical, but one possibility, compounds of fluorine, had not been considered. The element fluorine is a highly toxic and corrosive gas, and few organic compounds containing fluorine had ever been prepared.
Midgley and Henne decided to prepare a number of different molecules containing one or two carbon atoms and a varying number of fluorine and chlorine atoms instead of hydrogen atoms. The resulting compounds, chlorofluorocarbons (or CFCs, as they are now known), admirably fulfilled all the technical requirements of a refrigerant and were also very stable, nonflammable, nontoxic, inexpensive to manufacture, and nearly odorless.
In a very dramatic manner Midgley demonstrated the safety of his new refrigerants at a 1930 meeting of the American Chemical Society in Atlanta, Georgia. He poured some liquid CFC into an open container, and as the refrigerant boiled, he put his face in the vapor, opened his mouth, and took a deep breath. Turning to a previously lit candle he slowly exhaled the CFC, extinguishing the candle flameâa remarkable and unusual demonstration of the nonexplosive and nonpoisonous properties of this chlorofluorocarbon.
A number of different CFC molecules were then put into use as refrigerants: dichlorodifluoromethane, which was more usually known by its Du Pont Corporation trade name of Freon 12; trichlorofluoromethane, or Freon 11; and 1,2-dichloro-1,1,2,2,-tetrafluoroethane, or Freon 114.
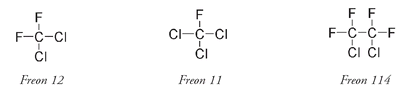
The numbers in the Freon names were a code developed by Midgley and Henne. The first digit is the number of carbon atoms minus one. If this is zero it does not get written; thus Freon 12 is really Freon 012. The next number is the number of hydrogen atoms (if any) plus one. The last number is the number of fluorine atoms. Any remaining atoms are chlorine.
CFCs were the perfect refrigerants. They revolutionized the refrigeration business and became the basis for a huge increase in home refrigeration especially as more and more homes were connected to electricity. By the 1950s a refrigerator was considered a standard home appliance in the developed world. Shopping for fresh foods on a daily basis was no longer necessary. Perishable items could be stored safely and meals readied ahead of time. The frozen food industry blossomed; new products were developed; ready-to-eat mealsâTV dinnersâwere introduced. CFCs changed how we bought food, how we prepared food, and even what food we ate. Refrigeration allowed heat-sensitive antibiotics, vaccines, and other medications to be stored and shipped around the world.
A plentiful supply of safe refrigerant molecules also gave people the means of cooling something other than foodâtheir surroundings. For centuries capturing natural breezes, moving air by means of fans, and using the cooling effect of evaporating water had been the main ways of coping with the temperature of hot climates. Once CFCs arrived on the scene, the fledgling air-conditioning industry expanded rapidly. In tropical regions and other places where summers were extremely hot, air-conditioning made homes, hospitals, offices, factories, malls, carsâanywhere people lived and workedâmore comfortable.
Other uses for CFCs were also found. As they reacted with virtually nothing, they made ideal propellants for virtually everything that could be applied through a spray can. Hair sprays, shaving foams, colognes, suntan lotions, whipped cream toppings, cheese spreads, furniture polish, carpet cleaners, bathtub mildew removers, and insecticides are just a few of the huge variety of products that were forced through the tiny holes of aerosol cans by expanding CFC vapor.
Some CFCs were perfect for foaming agents in the manufacture of the very light and porous polymers used as packing materials, as insulating foam in buildings, as fast food containers, and as “Styrofoam” coffee cups. The solvent properties of other CFCs, such as Freon 113, made them ideal cleaners for circuit boards and other electronic parts. Substitution of a bromine atom for a chlorine or a fluorine in the CFC molecule produced heavier compounds of higher boiling point, such as Freon 13B1 (the code is adjusted to indicate bromine), just right for use in fire extinguishers.

By the early 1970s almost a million tons of CFCs and related compounds were being produced annually. It seemed that these molecules were indeed ideal, perfectly suited to their roles in the modern world, without a drawback or a downside. They seemed to make the world a better place.
FREONS REVEAL THEIR DARK SIDEThe glow around CFCs lasted until 1974, when disturbing findings were announced by researchers Sherwood Rowland and Mario Molina at another meeting of the American Chemical Society in Atlanta. They had found that the very stability of CFCs presented a totally unexpected and extremely disturbing problem.
Unlike less stable compounds, CFCs don't break down by ordinary chemical reactions, a property that had originally made them so appealing. CFCs released into the lower atmosphere drift around for years or even decades, then eventually rise to the stratosphere, where they are ruptured by solar radiation. Within the stratosphere there is a stratum stretching from about fifteen to thirty kilometers above the surface of the earth known as the
ozone layer.
This may sound like a fairly thick cover, but if this same ozone layer were to exist at sea level pressures, it would measure only millimeters. In the rarefied region of the stratosphere, the air pressure is so low that the ozone layer is vastly expanded.
ozone layer.
This may sound like a fairly thick cover, but if this same ozone layer were to exist at sea level pressures, it would measure only millimeters. In the rarefied region of the stratosphere, the air pressure is so low that the ozone layer is vastly expanded.
Ozone is an elemental form of oxygen. The only difference between these forms is the number of atoms of oxygen in each moleculeâoxygen is O
2
and ozone is O
3
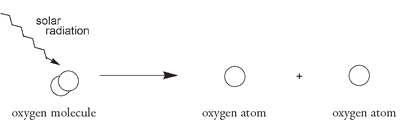
âbut the two molecules have very different properties. High above the ozone layer intense radiation from the sun breaks the bond in an oxygen molecule, producing two oxygen atoms:
2
and ozone is O
3
âbut the two molecules have very different properties. High above the ozone layer intense radiation from the sun breaks the bond in an oxygen molecule, producing two oxygen atoms:
Other books
The Wandering Caravan by E. L. Todd
The King Is Dead by Griff Hosker
Thanks for Giving by Chantal, Jillian
North Dakota Weddings by Elizabeth Goddard
Show Game: Story 2 of The Wife Games by Arla Coopa
Love Everlasting by Tracie Peterson
The Part Time People by Tom Lichtenberg, Benhamish Allen
Ordinary Life by Elizabeth Berg
Swansong by Damien Boyd
City of Ghosts by Stacia Kane