Penny le Couteur & Jay Burreson (32 page)
Read Penny le Couteur & Jay Burreson Online
Authors: Napoleon's Buttons: How 17 Molecules Changed History
Tags: #Philosophy & Social Aspects, #Science, #General, #World, #Chemistry, #Popular Works, #History

This, of course, was why prontosil red had been inactive in test tubes (in vitro) but not in live animals (in vivo). Sulfanilamide was found to be effective against many diseases other than streptococcal infections, including pneumonia, scarlet fever, and gonorrhea. Having recognized sulfanilamide as an antibacterial agent, chemists quickly started to synthesize similar compounds, hoping that slight modifications of the molecular structure would increase effectiveness and lessen any side effects. The knowledge that prontosil red was not the active molecule was extremely important. As can be seen from the structures, prontosil red is a more complicated molecule than sulfanilamide and it is more difficult to synthesize and to modify.
Between 1935 and 1946 more than five thousand variations of the sulfanilamide molecule were made. A number of them proved superior to sulfanilamide, whose side effects can include allergic responseârashes and feverâand kidney damage. The best results from varying the sulfanilamide structure were obtained when one of the hydrogen atoms of the SO
2
NH
2
was replaced with another group.
2
NH
2
was replaced with another group.

The resulting molecules are all part of the family of antibiotic drugs known collectively as
sulfanilamides
or
sulfa drugs.
A few of the many examples are
sulfanilamides
or
sulfa drugs.
A few of the many examples are
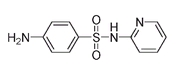
Sulfapyridineâused for pneumonia
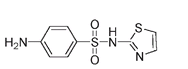
Sulfathiazoleâused for gastrointestinal infections
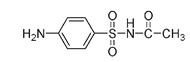
Sulfacetamideâused for urinary tract infections
Sulfa drugs were soon being described as wonder drugs and miracle cures. While such descriptives may seem unduly exaggerated nowadays, when numerous effective treatments against bacteria are available, the results obtained from these compounds in the early decades of the twentieth century appeared to be extraordinary. For example, after the introduction of sulfanilamides, the number of deaths from pneumonia dropped by twenty-five thousand a year in the United States alone.
In World War I, between 1914 and 1918, death from wound infection was as likely as death from injury on the battlefields of Europe. The major problem in the trenches and in any army hospital was a form of gangrene known as gas gangrene. Caused by a very virulent species of the
Clostridium
bacteria, the same genus responsible for the deadly botulism food poisoning, gas gangrene usually developed in deep wounds, typical of injuries from bombs and artillery where tissue was pierced or crushed. In the absence of oxygen, these bacteria multiply quickly. A brown foul-smelling pus is exuded, and gases from bacterial toxins bubble to the skin's surface, causing a distinctive stench.
Clostridium
bacteria, the same genus responsible for the deadly botulism food poisoning, gas gangrene usually developed in deep wounds, typical of injuries from bombs and artillery where tissue was pierced or crushed. In the absence of oxygen, these bacteria multiply quickly. A brown foul-smelling pus is exuded, and gases from bacterial toxins bubble to the skin's surface, causing a distinctive stench.
Before the development of antibiotics there was only one treatment for gas gangreneâamputation of the infected limb above the site of infection, in the hope of removing all the gangrenous tissue. If amputation was not possible, death was inevitable. During World War II, thanks to antibiotics such as sulfapyridine and sulfathiazoleâboth effective against gangreneâthousands of injured were spared disfiguring amputations, not to mention death.
We now know that the effectiveness of these compounds against bacterial infection has to do with the size and shape of the sulfanilamide molecule preventing bacteria from making an essential nutrient, folic acid. Folic acid, one of the B vitamins, is required for human cell growth. It is widely distributed in foods, such as leafy vegetables (hence the word
folic
from
foliage
), liver, cauliflower, yeast, wheat, and beef. Our bodies do not manufacture folic acid, so it is essential that we take it in with what we eat. Some bacteria, on the other hand, do not require supplemental folic acid, as they are able to make their own.
folic
from
foliage
), liver, cauliflower, yeast, wheat, and beef. Our bodies do not manufacture folic acid, so it is essential that we take it in with what we eat. Some bacteria, on the other hand, do not require supplemental folic acid, as they are able to make their own.
The folic acid molecule is fairly large and looks complicated:
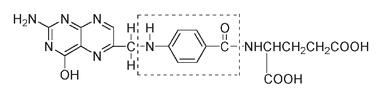
Folic acid with the middle portion from the
p
-aminobenzoic acid molecule outlined
p
-aminobenzoic acid molecule outlined
Consider just the part of its structure shown inside the outlined box in the structure above. This middle portion of the folic acid molecule is derived (in bacteria that make their own folic acid) from a smaller molecule,
p
-aminobenzoic acid.
p
-Aminobenzoic acid is thus an essential nutrient for these microorganisms.
p
-aminobenzoic acid.
p
-Aminobenzoic acid is thus an essential nutrient for these microorganisms.
The chemical structures of
p
-aminobenzoic acid and sulfanilamide are remarkably similar in shape and size, and it is this similarity that accounts for the antimicrobial activity of sulfanilamide. The lengths (as indicated by the square brackets) of each of these molecules measured from the hydrogen of the NH
2
group to the doubly bonded oxygen atom are within 3 percent of each other. As well they have almost the same width.
p
-aminobenzoic acid and sulfanilamide are remarkably similar in shape and size, and it is this similarity that accounts for the antimicrobial activity of sulfanilamide. The lengths (as indicated by the square brackets) of each of these molecules measured from the hydrogen of the NH
2
group to the doubly bonded oxygen atom are within 3 percent of each other. As well they have almost the same width.
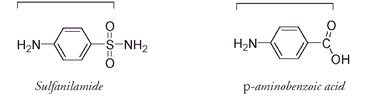
The bacterial enzymes involved in synthesizing folic acid appear to be unable to distinguish between the molecules of
p
-aminobenzoic acid that they need and the look-alike sulfanilamide molecules. Bacteria will thus unsuccessfully attempt to use sulfanilamide instead of
p
-aminobenzoic acidâand ultimately die because they are unable to make enough folic acid. We, relying on folic acid absorbed from our food, are not negatively affected by the action of sulfanilamide.
p
-aminobenzoic acid that they need and the look-alike sulfanilamide molecules. Bacteria will thus unsuccessfully attempt to use sulfanilamide instead of
p
-aminobenzoic acidâand ultimately die because they are unable to make enough folic acid. We, relying on folic acid absorbed from our food, are not negatively affected by the action of sulfanilamide.
Technically, sulfanilamide-based sulfa drugs are not true antibiotics. Antibiotics are properly defined as “substances of microbial origin that in very small amounts have antimicrobial activity.” Sulfanilamide is not derived from a living cell. It is man-made and is properly classified as an antimetabolite, a chemical that inhibits the growth of microbes. But the term
antibiotic
is now commonly used for all substances, natural or artificial, that kill bacteria.
antibiotic
is now commonly used for all substances, natural or artificial, that kill bacteria.
Although sulfa drugs were not the very first synthetic antibioticâthat honor belongs to Ehrlich's syphilis-fighting molecule salvarsanâthey were the first group of compounds that had widespread use in the fight against bacterial infection. Not only did they save the lives of hundreds of thousands of wounded soldiers and pneumonia victims, they were also responsible for an astounding drop in deaths of women in childbirth, because the streptococcus bacteria that cause puerperal or childbed fever also proved susceptible to sulfa drugs. More recently, however, the use of sulfa drugs has decreased worldwide, for a number of reasons: concern over their long-term side effects, the evolution of sulfanilamide-resistant bacteria, and the development of newer and more powerful antibiotics.
PENICILLINSThe earliest true antibiotics, from the penicillin family, are still in widespread use today. In 1877, Louis Pasteur was the first to demonstrate that one microorganism could be used to kill another. Pasteur showed that the growth of a strain of anthrax in urine could be prevented by the addition of some common bacteria. Subsequently Joseph Lister, having convinced the world of medicine of the value of phenol as an antiseptic, investigated the properties of molds, supposedly curing a persistent abscess in one of his patients with a compress soaked in a
Penicillium
-mold extract.
Penicillium
-mold extract.
Despite these positive results, further investigation of the curative properties of molds was sporadic until 1928, when a Scottish physician named Alexander Fleming, working at St. Mary's Hospital Medical School of London University, discovered that a mold of the
Penicillium
family had contaminated cultures of the staphylococci bacteria he was studying. He noted that a colony of the mold became transparent and disintegrated (undergoing what is called
lysis
). Unlike others before him Fleming was intrigued enough to follow through with further experimentation. He assumed that some compound produced by the mold was responsible for the antibiotic effect on the staphylococcus bacteria, and his tests confirmed this. A filtered broth, made from cultured samples of what we now know was
Penicillium notatum,
proved remarkably effective in laboratory tests against staphylococci grown in glass dishes. Even if the mold extract was diluted eight hundred times, it was still active against the bacterial cells. Moreover mice, injected with the substance that Fleming was now calling
penicillin,
showed no toxic effects. Unlike phenol, penicillin was nonirritating and could be applied directly to infected tissues. It also seemed to be a more powerful bacterial inhibitor than phenol. It was active against many bacteria species, including those causing meningitis, gonorrhea, and streptococcal infections like strep throat.
Penicillium
family had contaminated cultures of the staphylococci bacteria he was studying. He noted that a colony of the mold became transparent and disintegrated (undergoing what is called
lysis
). Unlike others before him Fleming was intrigued enough to follow through with further experimentation. He assumed that some compound produced by the mold was responsible for the antibiotic effect on the staphylococcus bacteria, and his tests confirmed this. A filtered broth, made from cultured samples of what we now know was
Penicillium notatum,
proved remarkably effective in laboratory tests against staphylococci grown in glass dishes. Even if the mold extract was diluted eight hundred times, it was still active against the bacterial cells. Moreover mice, injected with the substance that Fleming was now calling
penicillin,
showed no toxic effects. Unlike phenol, penicillin was nonirritating and could be applied directly to infected tissues. It also seemed to be a more powerful bacterial inhibitor than phenol. It was active against many bacteria species, including those causing meningitis, gonorrhea, and streptococcal infections like strep throat.
Other books
Big Book of Smut by Gia Blue
The Flight of the Iguana by David Quammen
FILLED By The Lusty Lords (Historical Smut With A Side Of Story) by Diana Quippley
Tying the Knot by Elizabeth Craig
The Alien in the Garage and Other Stories by Rob Keeley
Pretty Hurts by Shyla Colt
The Volcano Lover by Susan Sontag
Bold Counsel (The Trials of Sarah Newby) by Vicary, Tim
The Strip Club Dating Survival Guide by Jason Keeler
Galactic Alliance 3: Honor Thy Enemy by Doug Farren