Penny le Couteur & Jay Burreson (20 page)
Read Penny le Couteur & Jay Burreson Online
Authors: Napoleon's Buttons: How 17 Molecules Changed History
Tags: #Philosophy & Social Aspects, #Science, #General, #World, #Chemistry, #Popular Works, #History

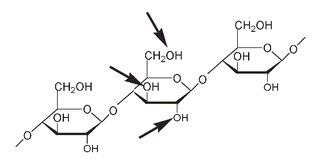
A portion of a cellulose molecule. The arrows on the middle glucose unit indicate the OH groups where nitration could take place on each glucose unit along the chain.
Chardonnet patented his process in 1885 and started manufacturing Chardonnet silk in 1891. But the flammability of the material proved to be its downfall. In one incident a cigar-smoking gentleman flicked ash on the Chardonnet silk dress of his dancing partner. The garment was reported to have disappeared in a flash of flame and a puff of smoke; there was no mention of the fate of the lady. Although this event and a number of other disasters at the Chardonnet factory led to its closure, Chardonnet did not give up on his artificial silk. By 1895 he was using a somewhat different process, involving a denitrating agent that produced a much safer cellulose-based artificial silk that was no more flammable than ordinary cotton.
Another method, developed in England, in 1901, by Charles Cross and Edward Bevan produced
viscose,
so named because of its high viscosity. When viscose liquid was forced through a spinnerette into an acid bath, cellulose was regenerated in the form of a fine filament called viscose silk. This process was used by both the American Viscose Company, formed in 1910, and by the Du Pont Fibersilk Company (later to become the Du Pont Corporation), formed in 1921. By 1938, 300 million pounds of viscose silk were being produced annually, supplying a growing demand for the new synthetic fabrics with the desired silky gloss so reminiscent of silk.
viscose,
so named because of its high viscosity. When viscose liquid was forced through a spinnerette into an acid bath, cellulose was regenerated in the form of a fine filament called viscose silk. This process was used by both the American Viscose Company, formed in 1910, and by the Du Pont Fibersilk Company (later to become the Du Pont Corporation), formed in 1921. By 1938, 300 million pounds of viscose silk were being produced annually, supplying a growing demand for the new synthetic fabrics with the desired silky gloss so reminiscent of silk.
The viscose process is still in use today, as the principal means of making what are now called rayonsâartificial silks, like viscose silk, in which the threads are composed of cellulose. Although it is still the same polymer of β-glucose units, the cellulose in rayon is regenerated under a slight tension, supplying a slight difference in twist to rayon threads that accounts for its high luster. Rayon, pure white in color and still having the same chemical structure, can be dyed to any number of tints and shades in the same manner as cotton. But it also has a number of drawbacks. While the pleated sheet structure of silk (flexible but resistant to stretching) makes it ideal for hosiery, the cellulose of rayon absorbs water, causing it to sag. This is not a desirable characteristic when used for stockings.
NYLON-A NEW ARTIFICIAL SILKA different type of artificial silk was needed, one that had rayon's good characteristics without its weaknesses. Noncellulose-based nylon arrived on the scene in 1938, created by an organic chemist hired by the Du Pont Fibersilk Company. By the late 1920s Du Pont had become interested in the plastic materials coming into the market. Wallace Carothers, a thirty-one-year-old organic chemist at Harvard University, was offered the opportunity to perform independent research for Du Pont on a virtually unlimited budget. He began work in 1928 at the new Du Pont laboratory dedicated to basic researchâitself a highly unusual concept, as within the chemical industry the practice of basic research was normally left to the universities.
Carothers decided that he wanted to work on polymers. At that time most chemists thought that polymers were actually groups of molecules clumped together and known as colloids; hence the name
collodion,
for the nitrocellulose derivative used in photography and in Chardonnet silk. Another opinion on the structure of polymers, championed by the German chemist Hermann Staudinger, was that these materials were extremely large molecules. The largest molecule synthesized up to that timeâby Emil Fischer, the great sugar chemistâhad a molecular weight of 4,200. In comparison, a simple water molecule has a molecular weight of 18, and a glucose molecule's molecular weight is 180. Within a year of starting work in the Du Pont laboratory, Carothers had made a polyester molecule with a molecular weight of over 5,000. He was then able to increase this value to 12,000, adding more evidence to the giant molecule theory of polymers, for which Staudinger was to receive the 1953 Nobel Prize in chemistry.
collodion,
for the nitrocellulose derivative used in photography and in Chardonnet silk. Another opinion on the structure of polymers, championed by the German chemist Hermann Staudinger, was that these materials were extremely large molecules. The largest molecule synthesized up to that timeâby Emil Fischer, the great sugar chemistâhad a molecular weight of 4,200. In comparison, a simple water molecule has a molecular weight of 18, and a glucose molecule's molecular weight is 180. Within a year of starting work in the Du Pont laboratory, Carothers had made a polyester molecule with a molecular weight of over 5,000. He was then able to increase this value to 12,000, adding more evidence to the giant molecule theory of polymers, for which Staudinger was to receive the 1953 Nobel Prize in chemistry.
Carothers's first polymer initially looked as if it had some commercial potential, as its long threads glistened like silk and did not become stiff or brittle on drying. Unfortunately, it melted in hot water, dissolved in common cleaning solvents, and disintegrated after a few weeks. For four years Carothers and his coworkers prepared different types of polymers and studied their properties, before they finally produced nylon, the man-made fiber that comes the closest to having the properties of silk and that deserves to be described as “artificial silk.”
Nylon is a polyamide, meaning that, as with silk, its polymer units are held together through amide linkages. But while silk has both an acid end and an amine end on each of its individual amino acid units, Carothers's nylon was made from two different monomer unitsâone with two acid groups and one with two amine groupsâalternating in the chain. Adipic acid has acid groups COOH at both ends:
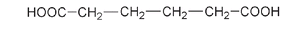
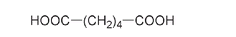
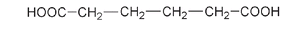
Structure of adipic acid, showing the two acid groups at each end of the molecule. The acid group -COOH is written backward as HOOC- when it is shown on the left-hand side.
or written as a condensed structure (below):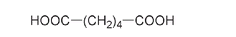
The condensed structure of the adipic acid molecule
The other molecular unit, 1,6-diaminohexane, has a very similar structure to that of adipic acid except there are amino groups (NH
2
) attached in place of the COOH acid groups. The structure and its condensed version are shown below:
2
) attached in place of the COOH acid groups. The structure and its condensed version are shown below:
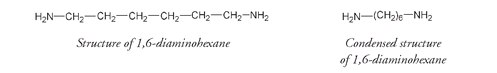
The amide link in nylon, like the amide link in silk, is formed by eliminating a molecule of water between the ends of the two molecules, from the H atom from NH
2
and the OH from COOH. The resulting amide bond, shown as -CO-NH- (or in reverse order as -NH-CO-) joins the two different molecules. It is in this respectâhaving the same amide linkâthat nylon and silk are chemically similar. In the making of nylon both the amino ends of 1,6-diaminohexane react with the acid ends of different molecules. This continues with alternating molecules adding to each end of a growing nylon chain. Carothers's version of nylon became known as “nylon 66” because each monomer unit has six carbon atoms.
2
and the OH from COOH. The resulting amide bond, shown as -CO-NH- (or in reverse order as -NH-CO-) joins the two different molecules. It is in this respectâhaving the same amide linkâthat nylon and silk are chemically similar. In the making of nylon both the amino ends of 1,6-diaminohexane react with the acid ends of different molecules. This continues with alternating molecules adding to each end of a growing nylon chain. Carothers's version of nylon became known as “nylon 66” because each monomer unit has six carbon atoms.
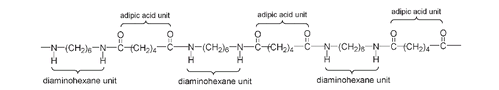
Structure of nylon, showing alternating molecules of adipic acid and 1,6-diaminohexane
The first commercial use of nylon, in 1938, was for toothbrush bristles. Then in 1939 nylon stockings were marketed for the first time. Nylon proved to be the ideal polymer for stockings. It had many of the desirable properties of silk; it did not sag and wrinkle like cotton or rayon; and most important, it was far less expensive than silk. Nylon hosiery was an enormous commercial success. In the year after they were introduced, some 64 million pairs of “nylons” were manufactured and sold. So overwhelming was the response to this product that the word
nylons
is now synonymous with women's hosiery. With its exceptional strength, durability, and lightness, nylon quickly found a use in many other products: fishing lines and nets, strings for tennis and badminton rackets, surgical sutures, and coatings for electrical wires.
nylons
is now synonymous with women's hosiery. With its exceptional strength, durability, and lightness, nylon quickly found a use in many other products: fishing lines and nets, strings for tennis and badminton rackets, surgical sutures, and coatings for electrical wires.
During World War II Du Pont's main production of nylon shifted from the fine filaments used in hosiery to the coarser yarns needed for military products. Tire cords, mosquito netting, weather balloons, ropes, and other military items dominated the use of nylon. In aviation nylon proved to be an excellent substitute for silk parachute shrouds. After the end of the war production in nylon plants was quickly converted back to civilian products. By the 1950s nylon's versatility was apparent in its use in clothing, skiwear, carpets, furnishings, sails, and many other products. It was also found to be an excellent molding compound and became the first “engineering plastic,” a plastic that is strong enough to be used as a replacement for metal. Ten million pounds of nylon were produced in 1953 for this use alone.
Unfortunately, Wallace Carothers did not live to see the success of his discovery. A victim of depression that became worse as he got older, he ended his life in 1937 by swallowing a vial of cyanide, unaware that the polymer molecule he had synthesized would play such a dominant role in the world of the future.
Â
Â
Silk and nylon share a similar legacy. It is more than just a comparable chemical structure and an eminent suitability for use in hosiery and parachutes. Both these polymers contributedâin their own wayâto enormous changes in the economic prosperity of their times. Not only did the demand for silk open worldwide trade routes and new trade agreements; it also led to the growth of cities that depended on silk production or the silk trade and helped establish other industries, such as dyeing, spinning, and weaving, that developed alongside sericulture. Silk brought great wealth and great change to many parts of the globe.
Just as silk and silk production stimulated fashionsâin clothing, furnishings, and artâin Europe and Asia for centuries, the introduction of nylon and a wealth of other modern textiles and materials has had a vast influence on our world. Where once plants and animals furnished the starting materials for our clothing, the raw products for many fabrics now come from by-products of oil refining. As a commodity, oil has taken over a position that once belonged to silk. As was once the case with silk, the demand for oil has forged new trade agreements, opened new trade routes, encouraged the growth of some cities and the establishment of others, created new industries and new jobs, and brought great wealth and great change to many parts of the globe.
Other books
The Roommate Situation by Zoe X. Rider
The Forget-Me-Not Summer by Leila Howland
Lead Me On by Julie Ortolon
Ambassador by William Alexander
The Forever Drug by Lisa Smedman
Demon's Fall by Lee, Karalynn
Broken Wings (The Broken Series Book 3) by Ruff, K.S.
Betrayal: Abby's Guilt (The Betrayal Series) by Velardi, Sofia
Cain's Salvation (Passion in Paradise - The Men of the McKinnon Sisters) by Sarah O'Rourke