Penny le Couteur & Jay Burreson (52 page)
Read Penny le Couteur & Jay Burreson Online
Authors: Napoleon's Buttons: How 17 Molecules Changed History
Tags: #Philosophy & Social Aspects, #Science, #General, #World, #Chemistry, #Popular Works, #History

These oxygen atoms float down to the ozone layer, where each reacts with another oxygen molecule to form ozone:
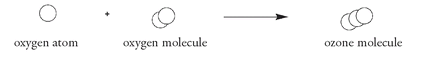
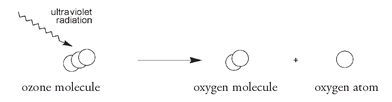
Within the ozone layer ozone molecules are broken up by high-energy ultraviolet radiation to form an oxygen molecule and an oxygen atom.
Two oxygen atoms now recombine to form the O
2
molecule:
2
molecule:
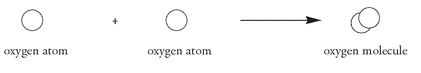
Thus in the ozone layer ozone is constantly being made and constantly being broken down. Over millennia these two processes have achieved a balance, so that the concentration of ozone in the Earth's atmosphere remains relatively constant. This arrangement has important consequences for life on earth; ozone in the ozone layer absorbs the portion of the ultraviolet spectrum from the sun that is most harmful to living things. It has been said that we live under an umbrella of ozone that protects us from the sun's deadly radiation.
But Rowland and Molina's research findings showed that chlorine atoms increase the rate of breakdown of ozone molecules. As a first step, a chlorine atom collides with ozone to form a chlorine monoxide molecule (ClO), and leaves behind an oxygen molecule:

In the next step ClO reacts with an oxygen atom to form an oxygen molecule and regenerates the chlorine atom:
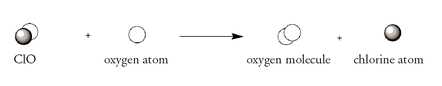
Rowland and Molina suggested that this overall reaction could upset the balance between ozone and oxygen molecules, as chlorine atoms hasten the breakup of ozone but have no effect on the making of ozone. A chlorine atom, used up in the first step of the ozone breakdown and produced anew in the second step, acts as a catalyst; that is, it increases the rate of reaction but is not itself consumed. This is the most alarming aspect of the effect of chlorine atoms on the ozone layerânot just that ozone molecules are being destroyed by chlorine but that the same chlorine atom can catalyze this breakdown again and again. One estimate is that, on average, every chlorine atom that finds its way to the upper atmosphere via a CFC molecule will destroy a hundred thousand ozone molecules before it is deactivated. For every 1 percent of ozone layer depletion, an additional 2 percent of damaging ultraviolet radiation might penetrate the Earth's atmosphere.
Based on their experimental results, Rowland and Molina predicted that chlorine atoms from CFCs and related compounds would, on reaching the stratosphere, start the decomposition of the ozone layer. At the time of their research billions of CFC molecules were being released into the atmosphere every day. The news that CFCs posed a real and immediate threat of depletion of the ozone layer and to the health and safety of all living things prompted some concerned reaction, but it was a number of yearsâand further studies, reports, task forces, voluntary phaseouts, and partial bansâbefore CFCs were completely banned.
Data from an entirely unexpected source provided the political will to ban CFCs. In 1985 studies from the Antarctic showed a growing depletion in the ozone layer above the South Pole. That the largest so-called “hole” in the ozone layer could appear in winter above a virtually uninhabited continentâthere was little call for refrigerants or aerosol hair sprays in Antarcticaâwas baffling. It obviously meant that the release of CFCs into the environment was a global concern and not just a local problem. In 1987 a high-altitude research plane flying above the south polar region found chlorine monoxide (ClO) molecules in the low-ozone areasâexperimental verification of the predictions of Rowland and Molina (who eight years later shared in the 1995 Nobel Prize in chemistry for their recognition of the long-term effects of CFCs on the stratosphere and the environment).
In 1987 an agreement called the Montreal Protocol required all the nations who signed it to commit to a phaseout of the use of CFCs and ultimately a complete ban. Today hydrofluorocarbon and hydrochlorofluorocarbon compounds are used as refrigerants instead of chlorofluorocarbons. These substances either do not contain chlorine or are more easily oxidized in the atmosphere; few reach the high stratospheric levels that the less reactive CFCs did. But the newer replacements for CFCs are not as effective refrigerants, and they require up to 3 percent more energy for the refrigeration cycle.
There are still billions of CFC molecules in the atmosphere. Not every country has signed the Montreal Protocol, and even in those countries that have there are still millions of CFC-containing refrigerators in use and probably hundreds of thousands of old abandoned appliances leaking CFCs into the atmosphere, where they will join the rest of the CFCs on the slow but inevitable journey upward to wreak havoc on the ozone layer. The effect of these once-lauded molecules may be felt for hundreds of years to come. If the intensity of high-energy ultraviolet radiation reaching the Earth's surface increases, the potential for damage to cells and their DNA moleculesâleading to higher levels of cancer and greater rates of mutationâalso increases.
THE DARK SIDE OF CHLORINEChlorofluorocarbons are not the only chemical group that were considered wonder molecules when first discovered but later revealed an unexpected toxicity or potential for environmental or social damage. What is perhaps surprising, however, is that organic compounds containing chlorine have shown this “dark side” more than any other group of organic compounds. Even elemental chlorine displays the dichotomy. Millions of people around the world depend on chlorination of their water supplies, and while other chemicals may be as effective as chlorine in purifying water, they are a lot more expensive.
One of the major public health advances of the past century has been the effort to bring clean drinking water to all parts of the worldâsomething we have still to achieve. Without chlorine we would be a lot further from this goal; yet chlorine is poisonous, a fact well understood by Fritz Haber, the German chemist whose work on synthesizing ammonia from nitrogen in the air, and on gas warfare, was described in Chapter 5. The first poisonous compound used in World War I was the yellowish-green chlorine gas, whose initial effects include choking and difficulty breathing. Chlorine is a powerful irritant to cells and can cause fatal swelling of tissues in the lungs and airways. Mustard gas and phosgene, compounds used in later poisonous gas releases, are also chlorine-containing organic compounds, with effects as horrifying as those of chlorine gas. Although the mortality rate for exposure to mustard gas is not high, it does cause permanent eye damage and severe, lasting respiratory impairment.
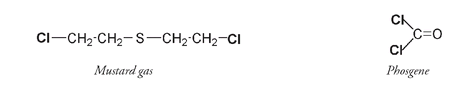
Poisonous gas molecules used in World War I. The chlorine atoms are bolded.
Phosgene gas is colorless and highly toxic. It is the most insidious of these poisons; it is not immediately irritating, so fatal concentrations may be inhaled before its presence is detected. Death usually results from severe swelling of tissues in the lungs and airways, which leads to suffocation.
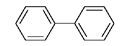
PCBS-FURTHER TROUBLE FROM CHLORINATED COMPOUNDSStill more chlorocarbon compounds that were initially greeted as wonder molecules have, like CFCs, turned out to pose a serious health hazard. Industrial production of polychlorinated biphenyls, or PCBs as they are most commonly known, began in the late 1920s. These compounds were considered ideal for use as electrical insulators and coolants in transformers, reactors, capacitors, and circuit breakers, where their extreme stability, even at high temperatures, and their lack of flammability were highly prized. They were employed as plasticizersâflexibility-enhancing agentsâin the manufacture of various polymers, including those used for wrapping in the food industry, for liners in baby bottles, and for polystyrene coffee cups. PCBs also found a use in the manufacture of various inks in the printing business, carbonless copying paper, paints, waxes, adhesives, lubricants, and vacuum pump oils.
Polychlorinated biphenyls are compounds where chlorine atoms have been substituted for hydrogen atoms on the parent biphenyl molecule.
Other books
Nocturne by Syrie James
Plague War by Jeff Carlson
A Merry Mistletoe Wedding by Judy Astley
Dead By Dusk by Heather Graham
The Education of a Traitor: A Memoir of Growing Up in Cold War Russia by Svetlana Grobman
The Chapel Perilous by Kevin Hearne
Hunted by the Alpha (Paranormal Werewolf Shifter Romance) by Reyer, Celina
Little Rainbows by Helena Stone
Damaged Goods by Stephen Solomita
Things My Girlfriend and I Have Argued About by Mil Millington