Penny le Couteur & Jay Burreson (49 page)
Read Penny le Couteur & Jay Burreson Online
Authors: Napoleon's Buttons: How 17 Molecules Changed History
Tags: #Philosophy & Social Aspects, #Science, #General, #World, #Chemistry, #Popular Works, #History

With salt in such demand, it is hardly surprising that it was often considered a prize of war rather than a commodity of trade. In ancient times settlements around the Dead Sea were conquered specifically for their precious supplies of salt. In the Middle Ages the Venetians waged war against neighboring coastal communities who threatened their all-important salt monopoly. Capturing an enemy's supply of salt was long considered a sound wartime tactic. During the American Revolution salt shortages resulted from a British embargo of imports from Europe and the West Indies into the former colony. The British destroyed salt works along the New Jersey coast to maintain the hardship affecting the colonists as a result of the high prices for imported salt. The 1864 capture of Saltville, Virginia, by Union forces during the American Civil War was seen as a vital step in reducing civilian morale and defeating the Confederate army.
It has even been suggested that a lack of dietary salt might have prevented wartime wounds from healing and was thus responsible for the death of thousands of Napoleon's soldiers during the 1812 retreat from Moscow. Lack of ascorbic acid (and the subsequent onset of scurvy) seems as likely a culprit as lack of salt under these circumstances, so both these compounds could join tin and lysergic acid derivatives as chemicals that thwarted Napoleon's dreams.
THE STRUCTURE OF SALTHalite, with a solubility of about 36 grams in every 100 grams of cold water, is far more soluble in water than are other minerals. As life is thought to have developed in the oceans and as salt is essential for life, without this solubility of salt life as we know it would not exist.
The Swedish chemist Svante August Arrhenius first proposed the idea of oppositely charged ions as an explanation for the structure and properties of salts and their solutions in 1887. For over a century scientists had been mystified by a particular property of salt solutionsâtheir ability to conduct electrical currents. Rainwater shows no electrical conductivity, yet saline solutions and solutions of other salts are excellent conductors. Arrhenius's theory accounted for this conductivity; his experiments showed that the more salt dissolves into solution, the greater the concentration of the charged speciesâthe ionsâneeded to carry the electrical current.
The concept of ions, as proposed by Arrhenius, also explained why acids, despite seemingly different structures, have similar properties. In water all acids produce hydrogen ions (H
+
) which are responsible for the sour taste and chemical reactivity of acid solutions. Although Arrhenius's ideas were not accepted by many conservative chemists of the time, he displayed a commendable degree of perseverance and diplomacy in campaigning determinedly for the soundness of the ionic model. His critics were eventually convinced, and Arrhenius received the 1903 Nobel Prize in chemistry for his electrolytic dissociation theory.
+
) which are responsible for the sour taste and chemical reactivity of acid solutions. Although Arrhenius's ideas were not accepted by many conservative chemists of the time, he displayed a commendable degree of perseverance and diplomacy in campaigning determinedly for the soundness of the ionic model. His critics were eventually convinced, and Arrhenius received the 1903 Nobel Prize in chemistry for his electrolytic dissociation theory.
By this time there was both a theory and practical evidence for how ions form. British physicist Joseph John Thomson in 1897 had demonstrated that all atoms contain
electrons,
the negatively charged fundamental particle of electricity that had been first proposed in 1833 by Michael Faraday. Thus if one atom lost an electron or electrons, it became a positively charged ion; if another atom gained an electron or electrons, a negatively charged ion was formed.
electrons,
the negatively charged fundamental particle of electricity that had been first proposed in 1833 by Michael Faraday. Thus if one atom lost an electron or electrons, it became a positively charged ion; if another atom gained an electron or electrons, a negatively charged ion was formed.
Solid sodium chloride is composed of a regular array of two different ionsâpositively charged sodium ions and negatively charged chloride ionsâheld together by strong attractive forces between the negative and positive charges.
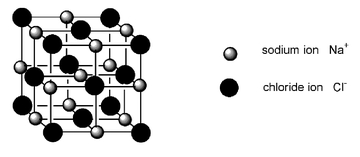
The three-dimensional structure of solid sodium chloride. The lines joining the ions are nonexistentâthey are included here to show the cubic arrangement of the ions.
Water molecules, although not consisting of ions, are partially charged. One side of a water molecule (the hydrogen side) is slightly positive, and the other side (the oxygen side) is slightly negative. This is what allows sodium chloride to dissolve in water. Although the attraction between a positive sodium ion and the negative end of water molecules (and the attraction between negative chloride ions and the positive end of water molecules) are similar to the attractive force between Na
+
ions and Cl- ions, what ultimately accounts for the solubility of salt is the tendency for these ions to disperse randomly. If ionic salts do not dissolve to any extent in water, it is because the attractive forces between the ions are greater than the water-to-ion attractions.
+
ions and Cl- ions, what ultimately accounts for the solubility of salt is the tendency for these ions to disperse randomly. If ionic salts do not dissolve to any extent in water, it is because the attractive forces between the ions are greater than the water-to-ion attractions.
Representing the water molecule as:
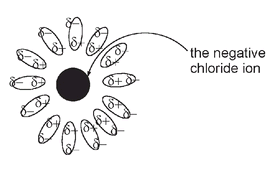
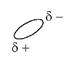 with δ- indicating the partial negative end of the molecule and δ+ the partial positive end of the molecule, we can show the negative chloride ions in aqueous solution as surrounded by the slightly positive end of water molecules:
with δ- indicating the partial negative end of the molecule and δ+ the partial positive end of the molecule, we can show the negative chloride ions in aqueous solution as surrounded by the slightly positive end of water molecules:
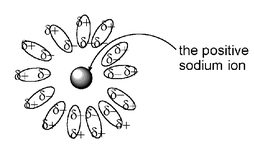
 and the positive sodium ion in aqueous solution as surrounded by the slightly negative end of water molecules:
and the positive sodium ion in aqueous solution as surrounded by the slightly negative end of water molecules:
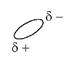


It is this solubility of sodium chloride that makes saltâby attracting water moleculesâsuch a good preservative. Salt preserves meat and fish by removing water from the tissues; in conditions of much-reduced water levels and a high salt content, the bacteria that cause decay are unable to survive. A lot more salt was used in this manner to keep food from decaying than was deliberately added to enhance flavors. In regions where dietary salt came mainly from meat, additional salt for food preservation was an essential factor in maintaining life. The other traditional methods of food preservation, smoking and drying, very often required the use of salt as part of the process. Food would be soaked in a brine solution prior to the actual smoking or drying. Communities without a local source of salt were dependent on supplies obtained by trade.
THE BODY'S NEED FOR SALTFrom earliest times, even if it was not needed for food preservation, humans recognized the necessity to obtain salt for their diet. Ions from salt play an essential role in the human body, maintaining the electrolyte balance between cells and the fluid surrounding the cells. Part of the process that generates the electrical impulses transmitted along neurons in the nervous system involves what is called the sodium-potassium pump. More Na
+
(sodium) ions are forced out of a cell than K
+
(potassium) ions are pumped into it, resulting in a net negative charge of the cytoplasm inside the cell compared with the outside of the cell membrane. Thus a difference in chargeâknown as a membrane potentialâis generated, which powers electrical impulses. Salt is therefore vital for the functioning of nerves and ultimately muscle movement.
+
(sodium) ions are forced out of a cell than K
+
(potassium) ions are pumped into it, resulting in a net negative charge of the cytoplasm inside the cell compared with the outside of the cell membrane. Thus a difference in chargeâknown as a membrane potentialâis generated, which powers electrical impulses. Salt is therefore vital for the functioning of nerves and ultimately muscle movement.
Cardiac glycoside molecules, such as the digoxin and digitoxin found in foxglove, inhibit the sodium-potassium pump, giving a higher level of Na
+
ions inside the cell. This ultimately increases the contractive force of the heart muscles and accounts for the activity of these molecules as heart stimulants. The chloride ion from salt is also needed in the body to produce hydrochloric acid, an essential component of the digestive juices in the stomach.
+
ions inside the cell. This ultimately increases the contractive force of the heart muscles and accounts for the activity of these molecules as heart stimulants. The chloride ion from salt is also needed in the body to produce hydrochloric acid, an essential component of the digestive juices in the stomach.
Salt concentration in a healthy person varies within a very narrow range. Lost salt must be replaced; excess salt must be excreted. Salt deprivation causes loss of weight and appetite, cramps, nausea, and inertia and can, in extreme cases of depletion of body saltâsuch as in marathon runnersâlead to vascular collapse and death. Excess sodium ion intake, however, is known to contribute to high blood pressure, a significant factor for cardiovascular disease, and to kidney and liver disorders.
The average human body contains about four ounces of salt; we are continuously losing salt, mainly through perspiration and excretion in urine, and so we have to replace it on a daily basis. Prehistoric man filled his dietary need for salt from the meat of the largely herbivorous animals he hunted, as raw meat is an excellent source of salt. As agriculture developed and grains and vegetables became a larger part of the diet, supplementary salt was needed. While carnivorous animals do not seek out salt licks, herbivorous animals need to do so. Humans in parts of the world where little meat is eaten and vegetarians require additional salt. Supplemental salt, a necessity as soon as humans adopted a settled agrarian way of life, must be obtained locally or through trade.
TAXING SALTThe human need for salt, together with its specific methods of production, have historically made this mineral peculiarly fitted for political control, monopoly, and taxation. For a government, a tax on salt would produce a reliable income. There was no substitute for salt, and everyone needed it, so everyone would have to pay. Salt sources were known; the production of salt is difficult to hide, salt itself is bulky and hard to conceal, and its transportation can be easily regulated and taxed. From 2000 B.C. in China, where the Emperor Hsia Yu ordered that the imperial court would be supplied by salt from Shantung Province, down through the ages, salt has been profitable for governments through taxes, tolls, and tariffs. In biblical times salt, considered a spice and taxed as such, was subject to customs duty at the many stopping places along caravan routes. After the death of Alexander the Great in 323 B.C., officials in Syria and Egypt continued to collect a salt tax that had originally been imposed by the Greek administration.
Throughout all these centuries the process of gathering taxes required tax collectors, many of whom became wealthy by increasing tax rates, adding extra duties, and selling exemptions. Rome was no exception. Originally the Ostia saltworks on the Tiber delta were taken over by the Roman state, so that salt could be supplied at reasonable rates to everyone. Such largesse did not last. The revenues from taxing salt offered too great a temptation, and a salt tariff was imposed. As the Roman Empire expanded, so did salt monopolies and salt taxes. Tax gatherers, independent agents supervised by the governor of each Roman province, levied taxes wherever they could. For those who lived far from salt-producing areas, the high cost of salt not only reflected transportation costs but tariffs, taxes, and duties at every step of the way.
Other books
The Blood Diamond by John Creasey
Once upon a Dream by Nora Roberts
Kill List (Special Ops #8) by Capri Montgomery
Why Now? by Carey Heywood
Keeping the Castle by Patrice Kindl
A King's Trade by Dewey Lambdin
Dark Fate: The Gathering (The Dark Fate Chronicles Book 1) by Matt Howerter, Jon Reinke
The Spook's Battle by Joseph Delaney
Stories in a Lost World: Bridget by Mortimer, L.C.
West of the Moon by Katherine Langrish