Penny le Couteur & Jay Burreson (53 page)
Read Penny le Couteur & Jay Burreson Online
Authors: Napoleon's Buttons: How 17 Molecules Changed History
Tags: #Philosophy & Social Aspects, #Science, #General, #World, #Chemistry, #Popular Works, #History

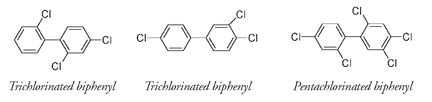
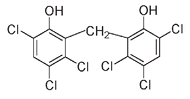
The biphenyl molecule
This structure has many possible arrangements, depending on how many chlorine atoms are present and where they are placed on the biphenyl rings. The following examples show two different trichlorinated biphenyls, each of which has three chlorines, and one pentachlorinated biphenyl with five chlorines. More than two hundred different combinations are possible.
Not long after the manufacture of PCBs began, reports of health problems among workers at PCB plants emerged. Many reported a skin condition now known as chloracne, where blackheads and suppurating pustules appear on the face and body. We now know that chloracne is one of the first symptoms of systemic PCB poisoning and can be followed by damage to the immune, nervous, endocrine, and reproductive systems, and by liver failure and cancer. PCBs are anything but a wonder molecule and, in fact, are among the most dangerous compounds ever synthesized. Their menace lies not only in their direct toxicity to humans and other animals but, like CFCs, in the very stability that made them so useful in the first place. PCBs persist in the environment; they are subject to the process of bioaccumulation (or biomagnification), where their concentration increases along the food chain. Animals at the top of the food chain, such as polar bears, lions, whales, eagles, and humans, can build up high concentrations of PCBs in the fat cells of their bodies.
In 1968 a devastating episode of human PCB poisoning epitomized the direct effects of ingestion of these molecules. Thirteen hundred residents of Kyushu, Japan, became illâinitially with chloracne and respiratory and vision problemsâafter eating rice-bran oil that had accidentally become contaminated with PCBs. The long-term consequences included birth defects and liver cancer fifteen times the normal rate. In 1977 the United States banned the discharge of PCB-containing materials into waterways. Their manufacture was finally outlawed in 1979, well after numerous studies had reported the toxic effects of these compounds on human health and the health of our planet. Despite regulations controlling PCBs, there are still millions of pounds of these molecules in use or awaiting safe disposal. They still leak into the environment.
CHLORINE IN PESTICIDES-FROM BOON TO BANE TO BANNEDOther chlorine-containing molecules have not just leaked into the environment; they have been deliberately put there in the form of pesticides, sometimes in huge amounts, over decades, and in many countries. Some of the most effective pesticides ever invented contain chlorine. Very stable pesticide moleculesâthose that persist in the environmentâwere originally thought to be desirable. The effects of one application could perhaps last for years. This indeed has turned out to be true, but unfortunately the consequences were not always as foreseen. The use of chlorine-containing pesticides has been of great value to humanity but has also caused, in some cases, totally unsuspected and very harmful side effects.
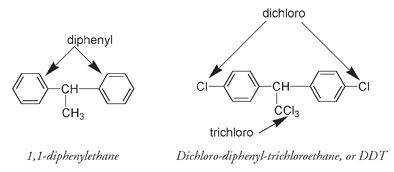
More than any other chlorine-containing pesticide, the DDT molecule illustrates the conflict between potential benefit and hazard. DDT is a derivative of 1,1-diphenylethane;
DDT
is an abbreviation from the name
dichloro-diphenyl-trichloroethane.
DDT
is an abbreviation from the name
dichloro-diphenyl-trichloroethane.
DDT was first prepared in 1874. That it was a potent insecticide was not realized until 1942, just in time for its use in World War II as a delousing powder to stop the spread of typhus and to kill the larvae of disease-carrying mosquitoes. “Bug bombs,” made from aerosol cans filled with DDT, were used extensively by the U.S. military in the South Pacific. These delivered a double blow to the environment, releasing large amounts of CFCs along with clouds of DDT.
Even before 1970, by which time three million tons of DDT had been manufactured and used, concerns about its effect on the environment and the development of insect resistance to it had surfaced. The effect of DDT on wildlife, particularly birds of prey such as eagles, falcons, and hawks that are at the top of food chains, are attributed not directly to DDT but instead to its main breakdown product. Both DDT and the breakdown product are fat-soluble compounds that accumulate in animal tissues. In birds, however, this breakdown product inhibits the enzyme that supplies calcium to their eggshells. Thus birds exposed to DDT will lay eggs with very fragile shells that often break before hatching. Starting in the late 1940s, a steep decline in the population of eagles, hawks, and falcons was noted. Major disturbances to the balance between useful and harmful insects, outlined by Rachel Carson in her 1962 book
Silent Spring,
were traced to increasingly heavy use of DDT.
Silent Spring,
were traced to increasingly heavy use of DDT.
During the Vietnam War, from 1962 to 1970, millions of gallons of Agent Orangeâa mixture of chlorine-containing herbicides 2,4-D and 2,4,5-Tâwere sprayed over areas of Southeast Asia to destroy guerrilla-concealing foliage.
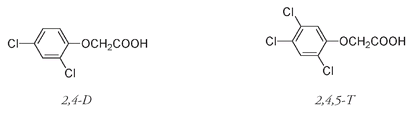
Although these two compounds are not particularly toxic, 2,4,5-T contains traces of a side product that has been implicated in the wave of birth defects, cancers, skin diseases, immune system deficiencies, and other serious health problems that affect Vietnam to this day. The compound responsible has the chemical name 2,3,7,8-tetrachlorodibenzodioxinânow commonly known as dioxin, though the word actually refers to a class of organic compounds that do not necessarily share the harmful properties of 2,3,7,8-tetrachlorodibenzodioxin.
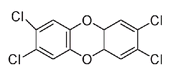
2,3,7,8-tetrachlorodibenzodioxin, or dioxin
Dioxin is considered the most lethal compound made by man, although it is still a million times less deadly than nature's most toxic compound, botulinum toxin A. In 1976 an industrial explosion in Seveso, Italy, allowed the release of a quantity of dioxin, with devastating resultsâchloracne, birth defects, cancerâfor local people and animals. Afterward widespread media reporting of the event firmly established all compounds referred to as dioxins as villains in the mind of the public.
Just as unexpected human health problems accompanied the use of a defoliant herbicide, so too did unexpected human health problems appear with another chlorinated molecule, hexachlorophene, an extremely effective germicide product used extensively in the 1950s and 1960s in soaps, shampoos, aftershave lotions, deodorants, mouthwashes, and similar products.
Hexachlorophene
Hexachlorophene was also routinely used on infants and added to diapers, talcum powders, and other baby toiletries. But in 1972 tests showed that its use led to brain and nervous system damage in laboratory animals. Hexachlorophene was subsequently banned from over-the-counter preparations and baby products, but because it is so effective against certain bacteria, it still has a limited use, despite its toxicity, in prescription acne medications and for surgical scrub preparations.
MOLECULES THAT PUT YOU TO SLEEPNot all chlorocarbon molecules have been disastrous for human health. Beyond the antiseptic properties of hexachlorophene, one small chlorine-containing molecule proved to be a boon for medicine. Until the mid- 1800s, surgery was performed without anesthesiaâbut sometimes with the administration of copious amounts of alcohol, in the belief that this would numb the agony. Some surgeons supposedly also imbibed in order to fortify themselves before inflicting such pain. Then in October 1846 a Boston dentist, William Morton, successfully demonstrated the use of ether as a way to induce narcosisâa temporary unconsciousnessâfor surgical procedures. Word of ether's ability to allow painless surgery spread rapidly, and other compounds were soon being investigated for anesthetic properties.
James Young Simpson, a Scottish physician and professor of medicine and midwifery at the University of Edinburgh Medical School, developed a unique way of testing compounds as possible anesthetics. He would allegedly ask his dinner guests to join him in inhaling various substances. Chloroform (CHCl
3
), first synthesized in 1831, evidently passed the test. Simpson came to on the dining-room floor after the experiment with this compound, surrounded by his still comatose visitors. Simpson lost no time in employing chloroform on his patients.
3
), first synthesized in 1831, evidently passed the test. Simpson came to on the dining-room floor after the experiment with this compound, surrounded by his still comatose visitors. Simpson lost no time in employing chloroform on his patients.
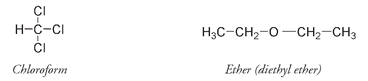
Other books
The Memory of Death by Trent Jamieson
Tossing the Caber (The Toss Trilogy) by Craig, Susan
Battlecruiser Alamo - 7 - Battlecruiser Alamo: Sacred Honor by Richard Tongue
Labyrinth by Tarah Scott
Tragedy's Gift: Surviving Cancer by Sharp, Kevin, Jeanne Gere
Serve His Needs - Destination by Karolyn James
Sex Secrets of an American Geisha by Conant, Py Kim
Shattered Illusions by Karen Michelle Nutt
Cinders and Ashes by King, Rebecca
Soul of Flame by Merryn Dexter