Penny le Couteur & Jay Burreson (50 page)
Read Penny le Couteur & Jay Burreson Online
Authors: Napoleon's Buttons: How 17 Molecules Changed History
Tags: #Philosophy & Social Aspects, #Science, #General, #World, #Chemistry, #Popular Works, #History

Throughout the Middle Ages in Europe the taxation of salt continued, often in the form of tolls imposed on barges or carts carrying salt from salt mines or coastal production plants. It reached its height in France with the infamous, oppressive, and much-hated salt tax known as the
gabelle.
Reports on the origin of the gabelle vary. Some accounts say Charles of Anjou in Provence imposed it in 1259, others that it started as a general tax applied to commodities like wheat, wine, and salt in the late thirteenth century to help maintain a permanent army. Whatever its true origin, by the fifteenth century the gabelle had become one of France's main national levies, and the name referred only to the tax on salt.
gabelle.
Reports on the origin of the gabelle vary. Some accounts say Charles of Anjou in Provence imposed it in 1259, others that it started as a general tax applied to commodities like wheat, wine, and salt in the late thirteenth century to help maintain a permanent army. Whatever its true origin, by the fifteenth century the gabelle had become one of France's main national levies, and the name referred only to the tax on salt.
But the gabelle was not just a tax on salt. It also carried the requirement that every man, woman, and child over the age of eight purchase a weekly amount of salt, at a price set by the king. Not only could the salt tax itself be raised, but the obligatory ration could also be increased at the monarch's whim. What was once intended as a uniform tax on the population soon extracted higher penalties on some regions of France than others. In general, those provinces that obtained their salt from Atlantic salt works were subject to the
grande gabelle,
more than twice what was paid in regionsâknown as Les Provinces des Petites Gabellesâwhere salt was supplied from Mediterranean saltworks. Through political influence or treaty arrangements some areas were exempt from the gabelle or paid only a fraction; at times there was no gabelle in Brittany and a special low rate in Normandy. At its height the gabelle increased the price of salt more than twenty times its real cost for those citizens in Les Provinces des Grandes Gabelles.
grande gabelle,
more than twice what was paid in regionsâknown as Les Provinces des Petites Gabellesâwhere salt was supplied from Mediterranean saltworks. Through political influence or treaty arrangements some areas were exempt from the gabelle or paid only a fraction; at times there was no gabelle in Brittany and a special low rate in Normandy. At its height the gabelle increased the price of salt more than twenty times its real cost for those citizens in Les Provinces des Grandes Gabelles.
Salt tax collectorsâreferred to as gabelle farmers, as they harvested the taxes from the peopleâwould monitor the per capita use of salt to ensure consumption obligations were met. Attempts to smuggle salt were rife despite severe penalties for being discovered with contraband salt; a common punishment in such cases was a sentence to the galleys. Peasant farmers and poor city dwellers were the most severely affected by the harsh and unfairly applied gabelle. Appeals to the king for relief from this onerous tax fell on deaf ears, and it has been suggested that the gabelle was one of the main grievances responsible for the French Revolution. It was abolished at the height of the revolution in 1790, and more than thirty gabelle collectors were executed. But the abolition did not last. In 1805 Napoleon reintroduced the gabelle, a measure he claimed was necessary to pay for his war against Italy. It was not finally eliminated until after World War II.
France was not the only country where such taxes on a necessity of life were burdensome. In coastal Scotland, particularly around the Firth of Forth, salt had been produced for centuries before it was ever taxed. Solar evaporation was not feasible in the cool, damp climate; seawater was boiled in large vessels, which were originally wood-fired and later coal-fired. By the 1700s there were more than 150 such saltworks in Scotland, plus numerous others that were peat-fired. The salt industry was so important to the Scots that the Eighth Article of the 1707 Treaty of Union between Scotland and England guaranteed Scotland a seven-year exemption from English salt taxes and after that a reduced rate forever. England's salt industry was based on the extraction of salt from brine as well as the mining of rock salt. Both methods were a great deal more efficient and profitable than the coal-fired seawater method of Scottish production. The industry in Scotland needed relief from English salt taxes in order to survive.
In 1825 the United Kingdom became the first country to abolish its salt tax, not so much because of the resentment this tax had generated among the working class throughout the centuries as because of the recognition of the changing role of salt. The Industrial Revolution is usually thought of as a mechanical revolutionâthe development of the flying shuttle, the spinning jenny, the water frame, the steam engine, the power loomâbut it was also a chemical revolution. Large-scale production of chemicals was required for the textile industry, for bleaching, soap making, glassmaking, potteries, the steel industry, tanneries, paper manufacturing, and the brewing and distilling industries. Manufacturers and factory owners pushed for the repeal of the salt tax because salt was becoming vastly more important as a starting material in manufacturing processes than as a preservative and food culinary supplement. Removal of the salt tax, sought by generations of the poor, became a reality only when salt was recognized as a key raw material for industrial prosperity in Britain.
Britain's enlightened stance on salt taxes did not extend to her colonies. In India a British-imposed salt tax became a symbol of colonial oppression seized upon by Mahatma Gandhi as he led the struggle for Indian independence. The salt tax in India was more than a tax. As many conquerors had found over the centuries, control of salt supplies meant political and economic control. Government regulations in British India made the nongovernmental sale or production of salt a criminal offense. Even collecting salt formed through natural evaporation around rock pools at the seacoast was outlawed. Salt, sometimes imported from England, had to be purchased from government agents at prices established by the British. In India, where the diet is mainly vegetarian and the often intensely hot climate promotes salt loss through sweating, it was especially important that salt be added to food. Under colonial rule the population was forced to pay for a mineral that millions had traditionally been able to gather or produce themselves at little or no cost.
In 1923, almost a century after Britain had eliminated the salt tax on its own citizens, the salt tax in India was doubled. In March 1930, Gandhi and a handful of supporters started a 240-mile march to the small village of Dandi, on the northwest coast of India. Thousands joined his pilgrimage, and once they reached the shoreline, they began to collect salt incrustations from the beach, to boil seawater, and to sell the salt they produced. Thousands more joined in breaking the salt laws; illegal salt was sold in villages and cities all over India and was frequently confiscated by the police. Gandhi's supporters were often brutalized by the police, and thousands were imprisoned. Thousands more took their places making salt. Strikes, boycotts, and demonstrations followed. By the following March the draconian salt laws of India had been modified: local people were permitted to collect salt or make it from local sources and sell it to others in their villages. Although a commercial tax still applied, the British government's salt monopoly was broken. Gandhi's ideals of nonviolent civil disobedience had proven effective, and the days of the British Raj were numbered.
SALT AS A STARTING MATERIALThe removal of the salt tax in Britain was important not only to those industries that used salt as part of their manufacturing processes but also to companies that made inorganic chemicals, where salt was a major starting material. It was particularly significant for another sodium compound, sodium carbonate (Na
2
CO
3
), known as soda ash or washing soda. Soda ash, used in soap making and needed in large quantities as the demand for soap increased, came mainly from naturally occurring deposits, often incrustations around drying alkaline lakes or from residues from burning kelp and other seaweeds. Soda ash from these sources was impure and supplies were limited, so the possibility of producing sodium carbonate from the plentiful supplies of sodium chloride attracted attention. In the 1790s Archibald Cochrane, the ninth earl of Dundonaldânow considered one of the leaders of Britain's chemical revolution and a founder of the chemical alkali industryâwhose modest family estate on Scotland's Firth of Forth bordered on numerous coal-fired saltpans, took out a patent for converting salt to “artificial alkali,” but his process was never a commercial success. In France in 1791, Nicolas Leblanc developed a method of making sodium carbonate from salt, sulfuric acid, coal, and limestone. The onset of the French Revolution delayed establishment of Leblanc's process, and it was in England where the profitable manufacture of soda ash began.
2
CO
3
), known as soda ash or washing soda. Soda ash, used in soap making and needed in large quantities as the demand for soap increased, came mainly from naturally occurring deposits, often incrustations around drying alkaline lakes or from residues from burning kelp and other seaweeds. Soda ash from these sources was impure and supplies were limited, so the possibility of producing sodium carbonate from the plentiful supplies of sodium chloride attracted attention. In the 1790s Archibald Cochrane, the ninth earl of Dundonaldânow considered one of the leaders of Britain's chemical revolution and a founder of the chemical alkali industryâwhose modest family estate on Scotland's Firth of Forth bordered on numerous coal-fired saltpans, took out a patent for converting salt to “artificial alkali,” but his process was never a commercial success. In France in 1791, Nicolas Leblanc developed a method of making sodium carbonate from salt, sulfuric acid, coal, and limestone. The onset of the French Revolution delayed establishment of Leblanc's process, and it was in England where the profitable manufacture of soda ash began.
In Belgium in the early 1860s, the brothers Ernest and Alfred Solvay developed an improved method of converting sodium chloride to sodium carbonate using limestone (CaCO
3
) and ammonia gas (NH
3
). The key steps were the formation of a precipitate of sodium bicarbonate (NaHCO
3
) from a concentrated solution of brine, infused with ammonia gas and carbon dioxide (from limestone):
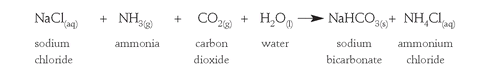 and then production of sodium carbonate by heating the sodium bicarbonate:
and then production of sodium carbonate by heating the sodium bicarbonate:
3
) and ammonia gas (NH
3
). The key steps were the formation of a precipitate of sodium bicarbonate (NaHCO
3
) from a concentrated solution of brine, infused with ammonia gas and carbon dioxide (from limestone):
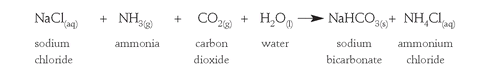
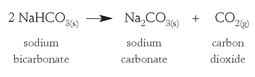
Today the Solvay process remains the main method of preparing synthetic soda ash, but discoveries of massive deposits of natural soda ashâthe Green River basin of Wyoming, for example, has soda ash resources estimated at over ten billion tonsâhave decreased the demand for its preparation from salt.
Another sodium compound, caustic soda (NaOH), has also long been in demand. Industrially, caustic soda or sodium hydroxide is made by passing an electric current through a solution of sodium chlorideâa process known as
electrolysis.
Caustic soda, one of the ten most produced chemicals in the United States, is essential in extracting aluminum metal from its ore and in the manufacture of rayon, cellophane, soaps, detergents, petroleum products, paper, and pulp. Chlorine gas, also produced in the electrolysis of brine, was originally considered a by-product of the process, but it was soon discovered that chlorine was an excellent bleaching agent and a potent disinfectant. Today the production of chlorine is as much a reason for commercial electrolysis of NaCl solutions as the production of NaOH. Chlorine is now used in the manufacture of many organic products, such as pesticides, polymers, and pharmaceuticals.
electrolysis.
Caustic soda, one of the ten most produced chemicals in the United States, is essential in extracting aluminum metal from its ore and in the manufacture of rayon, cellophane, soaps, detergents, petroleum products, paper, and pulp. Chlorine gas, also produced in the electrolysis of brine, was originally considered a by-product of the process, but it was soon discovered that chlorine was an excellent bleaching agent and a potent disinfectant. Today the production of chlorine is as much a reason for commercial electrolysis of NaCl solutions as the production of NaOH. Chlorine is now used in the manufacture of many organic products, such as pesticides, polymers, and pharmaceuticals.
From fairy tales to biblical parables, from Swedish folk myths to North American Indian legends, different societies around the world tell stories of salt. Salt is used in ceremonies and rites, it symbolizes hospitality and good fortune, and it protects against evil spirits and ill luck. The important role of salt in shaping human culture is also seen in language. We earn a salaryâthe derivation of the word comes from the fact that Roman soldiers were often paid in salt. Our words for salad (originally dressed only with salt), sauce and salsa, sausage and salami all come from the same Latin root. As in other languages, our everday speech is “salted” with metaphors: “salt of the earth,” “old salt,” “worth his salt,” “below the salt,” “with a grain of salt,” “back to the salt mine.”
The ultimate irony in the story of salt is that despite all the wars fought over it, despite the battles and protests over taxation and tolls imposed on it, despite migrations in search of it and the despair of hundreds of thousands imprisoned for smuggling it, by the time the discovery of new underground salt deposits and modern technology had vastly decreased its price, the need for salt in food preservation was already greatly diminishedârefrigeration had become the standard method of preventing decomposition of food. This compound that throughout history has been honored and revered, desired and fought over, and sometimes been valued more highly than gold, is nowadays not only cheap and readily available but is considered commonplace.
16. CHLOROCARBON COMPOUNDS
I
N 1877 THE ship
Frigorifique
sailed from Buenos Aires to the French port of Rouen with a cargo of Argentinian beef in its hold. While today this passage would be seen as routine, it was in fact a historic voyage. The ship carried a cooled cargo that signaled the beginning of the era of refrigeration and the end of food preservation by molecules of spice and salt.
KEEPING COOLN 1877 THE ship
Frigorifique
sailed from Buenos Aires to the French port of Rouen with a cargo of Argentinian beef in its hold. While today this passage would be seen as routine, it was in fact a historic voyage. The ship carried a cooled cargo that signaled the beginning of the era of refrigeration and the end of food preservation by molecules of spice and salt.
Since at least 2000 B.C., people have used ice to keep things cool, relying on the principle that solid ice absorbs heat from its surroundings as it melts. The liquid water produced drains away, and a new supply of ice is added. Refrigeration, on the other hand, involves not solid and liquid phases but liquid and vapor phases. As liquid evaporates, it absorbs heat from its surroundings. The vapor produced by evaporation is then returned to the liquid state by compression. This compression stage is what puts the
re
in
refrigeration
âvapor is returned to a liquid, then re-evaporates causing cooling, and the whole cycle repeats. A key component of the cycle is an energy source to drive the mechanical compressor. The old fashioned icebox, where ice had to be added continually, was technically not a refrigerator. Today we often use the word
refrigerate
to mean “to make or keep cool” without considering how it is done.
re
in
refrigeration
âvapor is returned to a liquid, then re-evaporates causing cooling, and the whole cycle repeats. A key component of the cycle is an energy source to drive the mechanical compressor. The old fashioned icebox, where ice had to be added continually, was technically not a refrigerator. Today we often use the word
refrigerate
to mean “to make or keep cool” without considering how it is done.
Other books
Love and Law by K. Webster
Rorey's Secret by Leisha Kelly
Daysider (Nightsiders) by Krinard, Susan
Gravity Box and Other Spaces by Mark Tiedemann
The Curse of a Single Red Rose (Haunted Hearts Series Book 7) by Denise Moncrief
Fortune's Formula by William Poundstone
Scandal's Reward by Jean R. Ewing
(You) Set Me on Fire by Mariko Tamaki
Lila's Thunder: The Almeida Brothers, Book One by Burns, Trevion