Penny le Couteur & Jay Burreson (24 page)
Read Penny le Couteur & Jay Burreson Online
Authors: Napoleon's Buttons: How 17 Molecules Changed History
Tags: #Philosophy & Social Aspects, #Science, #General, #World, #Chemistry, #Popular Works, #History

Rubber and rubber products are so common that we probably never think about what rubber is and how it has changed our lives. Although mankind has been aware of its existence for hundreds of years, only in the last century and a half has rubber become an essential component of civilization. Its chemical structure gives rubber its unique properties, and the chemical manipulation of this structure produced a molecule from which fortunes have been made, lives have been lost, and countries have been changed forever.
ORIGINS OF RUBBERSome form of rubber was long known throughout most of Central and South America. The first use of rubber, for both decorative and practical purposes, is often attributed to Indian tribes of the Amazon basin. Rubber balls from a Mesoamerican archaeological site near Veracruz, Mexico, date back to between 1600 and 1200 B.C. On his second voyage to the New World, in 1495, Christopher Columbus saw Indians on the island of Hispaniola playing with heavy balls made of a plant gum that bounced surprisingly high. “Better than those fill'd with Wind in Spain,” he reported, presumably referring to the inflated animal bladders used by the Spanish for ball games. Columbus brought some of this new material back to Europe, as did other travelers to the New World after him. The samples of rubber latex remained mainly a novelty item, however; they became sticky and smelly during hot weather and hard and brittle in European winters.
A Frenchman named Charles-Marie de La Condamine was the first to investigate whether there was a serious use for this strange substance. La Condamineâvariously described as a mathematician, a geographer, and an astronomer, as well as a playboy and an adventurerâhad been sent by the French Academy of Sciences to measure a meridian through Peru to help in determining whether the Earth was actually slightly flattened at the poles. After completing his work for the academy, La Condamine took the opportunity to explore the South American jungle, arriving back in Paris in 1735 with a number of balls of the coagulated gum from the
caoutchouc
tree (the “tree that weeps”). He had observed the Omegus Indians of Ecuador collecting the sticky white caoutchouc sap, then holding it over a smoky fire and molding it into a variety of shapes to make containers, balls, hats, and boots. Unfortunately, La Condamine's samples of raw sap, which remained as latex when not preserved through smoking, fermented during shipping and arrived in Europe as a useless smelly mass.
caoutchouc
tree (the “tree that weeps”). He had observed the Omegus Indians of Ecuador collecting the sticky white caoutchouc sap, then holding it over a smoky fire and molding it into a variety of shapes to make containers, balls, hats, and boots. Unfortunately, La Condamine's samples of raw sap, which remained as latex when not preserved through smoking, fermented during shipping and arrived in Europe as a useless smelly mass.
Latex is a colloidal emulsion, a suspension of natural rubber particles in water. Many tropical trees and shrubs produce latex, including
Ficus elastica,
the houseplant usually referred to as the “rubber plant.” In parts of Mexico latex is still harvested in the traditional manner from wild rubber trees,
Castilla elastica.
All members of the widely distributed
Euphorbia
(milkweed or spurge) family are latex producers, including the familiar Christmas poinsettia, the cactuslike succulent
Euphorbias
from desert regions, deciduous and evergreen shrubby
Euphorbias,
and “Snow-on-the-Mountain,” an annual, fast-growing North American
Euphorbia. Parthenium argentatum,
or guayule, a shrub that grows in the southern United States and northern Mexico, also produces much natural rubber. Though it is neither tropical nor a
Euphorbia,
the humble dandelion is yet another latex producer. The single greatest producer of natural rubber is a tree that originated in the Amazon region of Brazil,
Hevea brasiliensis.
CIS AND TRANSFicus elastica,
the houseplant usually referred to as the “rubber plant.” In parts of Mexico latex is still harvested in the traditional manner from wild rubber trees,
Castilla elastica.
All members of the widely distributed
Euphorbia
(milkweed or spurge) family are latex producers, including the familiar Christmas poinsettia, the cactuslike succulent
Euphorbias
from desert regions, deciduous and evergreen shrubby
Euphorbias,
and “Snow-on-the-Mountain,” an annual, fast-growing North American
Euphorbia. Parthenium argentatum,
or guayule, a shrub that grows in the southern United States and northern Mexico, also produces much natural rubber. Though it is neither tropical nor a
Euphorbia,
the humble dandelion is yet another latex producer. The single greatest producer of natural rubber is a tree that originated in the Amazon region of Brazil,
Hevea brasiliensis.
Natural rubber is a polymer of the molecule isoprene. Isoprene, with only five carbon atoms, is the smallest repeating unit of any natural polymer, making rubber the simplest natural polymer. The first chemical experiments on the structure of rubber were conducted by the great English scientist Michael Faraday. Nowadays more often considered a physicist than a chemist, Faraday thought of himself a “natural philosopher,” the boundaries between chemistry and physics being less distinct during his time. Though he is mainly remembered for his physics discoveries in electricity, magnetism, and optics, his contributions to the field of chemistry were substantial and included establishing the chemical formula of rubber as a multiple of C
5
H
8
in 1826.
5
H
8
in 1826.
By 1835 it had been shown that isoprene could be distilled from rubber, suggesting that it was a polymer of repeating C
5
H
8
or isoprene units. Some years later this was confirmed when isoprene was polymerized to a rubberlike mass. The structure of the isoprene molecule is usually written as with two double bonds on adjacent carbon atoms. But rotation occurs freely around any single bond between two carbon atoms, as shown.
5
H
8
or isoprene units. Some years later this was confirmed when isoprene was polymerized to a rubberlike mass. The structure of the isoprene molecule is usually written as with two double bonds on adjacent carbon atoms. But rotation occurs freely around any single bond between two carbon atoms, as shown.
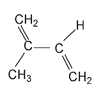

So these two structuresâand all the other possible twistings around this single bondâare still the same compound. Natural rubber is formed when isoprene molecules add to one another in an end-to-end fashion. This
polymerization
in rubber produces what are called
cis
double bonds. A double bond supplies rigidity to a molecule by preventing rotation. The result of this is that the structure on the left, below, known as the
cis
form, is not the same as the structure on the right, known as the
trans
form.
polymerization
in rubber produces what are called
cis
double bonds. A double bond supplies rigidity to a molecule by preventing rotation. The result of this is that the structure on the left, below, known as the
cis
form, is not the same as the structure on the right, known as the
trans
form.
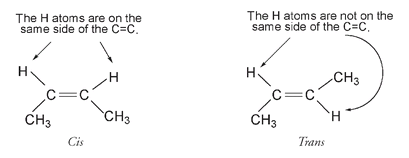
For the cis structure, the two H atoms (and also the two CH
3
groups) are both on the same side of the double bond, whereas in the trans structure the two H atoms (and also the two CH
3
groups) are on different sides of the double bond. This seemingly small difference in the way various groups and atoms are arranged around the double bond has enormous consequences for the properties of the different polymers from the isoprene molecule. Isoprene is only one of many organic compounds with cis and trans forms; they often have quite different properties.
3
groups) are both on the same side of the double bond, whereas in the trans structure the two H atoms (and also the two CH
3
groups) are on different sides of the double bond. This seemingly small difference in the way various groups and atoms are arranged around the double bond has enormous consequences for the properties of the different polymers from the isoprene molecule. Isoprene is only one of many organic compounds with cis and trans forms; they often have quite different properties.
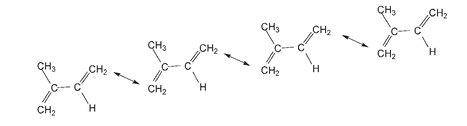
Below four isoprene molecules are shown ready to link up end to end, as indicated with the double-headed arrows, to form the natural rubber molecule.
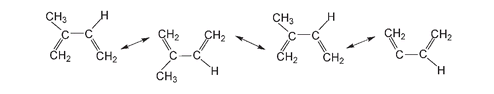
In the next drawing, the dashed lines indicate where the chain continues with the polymerization of further isoprene molecules.
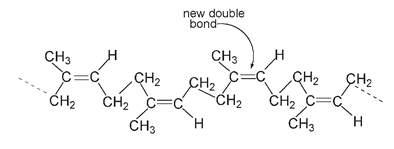
Natural rubber
New double bonds form when isoprene molecules combine; they are all cis with respect to the polymer chain, that is, the continuous chain of carbon atoms that makes the rubber molecule is on the same side of each double bond.
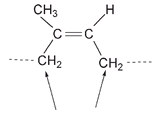
The carbons of the continuous chain are on the same side of this double bond, so this is a cis structure.
This cis arrangement is essential for the elasticity of rubber. But natural polymerization of the isoprene molecule is not always cis. When the arrangement around the double bond in the polymer is trans, another natural polymer with very different properties from those of rubber is produced. If we use the same isoprene molecule but twisted to the position shown,
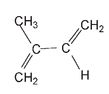 and then have four molecules like this add in an end-to-end fashion, joining as indicated again by double-headed arrows;
and then have four molecules like this add in an end-to-end fashion, joining as indicated again by double-headed arrows;
 the result is the trans product.
the result is the trans product.


Other books
Hard and Fast by Erin McCarthy
Death by Sarcasm by Dani Amore
The Way I Like It (The Soldiers of Wrath MC, #5) by Jenika Snow, Sam Crescent
Husband for Hire by Susan Crosby
The Ashes of London by Andrew Taylor
Our First Love by Anthony Lamarr
The Problem of Slavery in the Age of Emancipation by Davis, David Brion
A Sixpenny Christmas by Katie Flynn
Rome: An Empire's Story by Greg Woolf