Penny le Couteur & Jay Burreson (28 page)
Read Penny le Couteur & Jay Burreson Online
Authors: Napoleon's Buttons: How 17 Molecules Changed History
Tags: #Philosophy & Social Aspects, #Science, #General, #World, #Chemistry, #Popular Works, #History

Indigo and Tyrian purple were manufactured by these labor-intensive methods for centuries. It was not until the end of the nineteenth century that a synthetic form of indigo became available. In 1865 the German chemist Johann Friedrich Wilhelm Adolf von Baeyer began investigating the structure of indigo. By 1880 he had found a way to make it in the laboratory from easily obtainable starting materials. It took another seventeen years, however, before synthetic indigo, prepared by a different route and marketed by the German chemical company Badische Anilin und Soda Fabrik (BASF), became commercially viable.

Baeyer's first synthesis of indigo required seven separate chemical reactions.
This was the beginning of the decline of the large natural indigo industry, a change that altered the way of life for thousands whose livelihood depended on the cultivation and extraction of natural indigo. Today an annual production of over fourteen thousand tons makes synthetic indigo a major industrial dye. Though synthetic indigo (like the natural compound) notoriously lacks colorfastness, it is most often used to dye blue jeans, where this property is considered a fashion advantage. Millions of pairs of jeans are now made from specially pre-faded indigo-dyed denim. Tyrian purple, the dibromo derivative of indigo, was also produced synthetically through a process similar to indigo synthesis, although other purple dyes have superseded it.
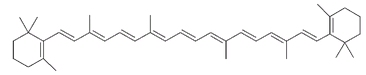
Dyes are colored organic compounds that are incorporated into the fibers of textiles. The molecular structure of these compounds allows the absorption of certain wavelengths of light from the visible spectrum. The actual color of the dye we see depends on the wavelengths of the visible light that is reflected back rather than absorbed. If all the wavelengths are absorbed, no light is reflected, and the color of the dyed cloth we see is black; if no wavelengths are absorbed, all light is reflected and the color we see is white. If only the wavelengths of red light are absorbed, then the reflected light is the complementary color of green. The relationship between the wavelength absorbed and the chemical structure of the molecule is very similar to the absorption of ultraviolet rays by sunscreen productsâthat is, it depends on the presence of double bonds alternating with single bonds. But for the absorbed wavelength to be in the visible range rather than the ultraviolet, there must be a greater number of these alternating double and single bonds. This is shown in the molecule β-carotene, below, which is responsible for the orange color of carrots, pumpkin, and squash.
β
-carotene (orange)
-carotene (orange)
Such alternating double and single bonds, as shown in carotene, are referred to as
conjugated.
β-carotene has eleven of these conjugated double bonds. Conjugation can be extended and the wavelength of absorbed light changed, when atoms such as oxygen, nitrogen, sulfur, bromine, or chlorine are also part of the alternating system.
conjugated.
β-carotene has eleven of these conjugated double bonds. Conjugation can be extended and the wavelength of absorbed light changed, when atoms such as oxygen, nitrogen, sulfur, bromine, or chlorine are also part of the alternating system.
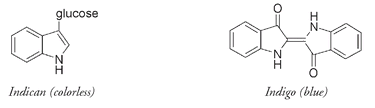
The indican molecule, from indigo and woad plants, has some conjugation but not enough to appear colored. The indigo molecule, however, has twice indican's number of alternating single and double bonds and also has oxygen atoms as part of the conjugation combination. It thus has enough to absorb light from the visible spectrum, which is why indigo is highly colored.
Distinct from organic dyes, finely ground minerals and other inorganic compounds have also been used since antiquity to create color. But while the color of these
pigments
âfound in cave drawings, tomb decorations, paintings, wall murals, and frescoesâis also due to absorption of certain wavelengths of visible light, it has nothing to do with conjugated double bonds.
pigments
âfound in cave drawings, tomb decorations, paintings, wall murals, and frescoesâis also due to absorption of certain wavelengths of visible light, it has nothing to do with conjugated double bonds.
The two common ancient dyes that were used for red shades have very different sources but surprisingly similar chemical structures. The first of these comes from the root of the madder plant. Madder plants, belonging to the
Rubiaceae
family, contain a dye called alizarin. Alizarin was probably first used in India, but it was also known in Persia and Egypt long before its use by the ancient Greeks and Romans. It is a mordant dye, that is, a dye where another chemicalâa metal ionâis needed to fix color to a fabric. Different colors can be obtained by first treating the fabric with different metal salt mordant solutions. The aluminum ion as a mordant produces a rose-red color; a magnesium mordant gives a violet color; chromium a brownish-violet; and calcium a reddish-purple. The bright red color obtained when both aluminum and calcium ions are in the mordant would have resulted from using clay with dried, crushed, and powdered madder root in the dye process. This is likely the dye/mordant combination used by Alexander the Great in 320 B.C., in a ruse to lure his enemy into an unnecessary battle. Alexander had his soldiers dye their uniforms with large patches of a blood-red dye. The attacking Persian army, assuming they were assaulting wounded survivors and expecting little resistance, were easily defeated by the smaller number of Alexander's soldiersâand, if the story is true, by the alizarin molecule.
Rubiaceae
family, contain a dye called alizarin. Alizarin was probably first used in India, but it was also known in Persia and Egypt long before its use by the ancient Greeks and Romans. It is a mordant dye, that is, a dye where another chemicalâa metal ionâis needed to fix color to a fabric. Different colors can be obtained by first treating the fabric with different metal salt mordant solutions. The aluminum ion as a mordant produces a rose-red color; a magnesium mordant gives a violet color; chromium a brownish-violet; and calcium a reddish-purple. The bright red color obtained when both aluminum and calcium ions are in the mordant would have resulted from using clay with dried, crushed, and powdered madder root in the dye process. This is likely the dye/mordant combination used by Alexander the Great in 320 B.C., in a ruse to lure his enemy into an unnecessary battle. Alexander had his soldiers dye their uniforms with large patches of a blood-red dye. The attacking Persian army, assuming they were assaulting wounded survivors and expecting little resistance, were easily defeated by the smaller number of Alexander's soldiersâand, if the story is true, by the alizarin molecule.
Dyes have long been associated with army uniforms. The blue coats supplied by France to the Americans during the American Revolution were dyed with indigo. The French army used an alizarin dye, known as Turkey red as it had been grown for centuries in the eastern Mediterranean, although it probably originated in India and gradually moved westward through Persia and Syria to Turkey. The madder plant was introduced into France in 1766, and by the end of the eighteenth century it had become one of the country's most important sources of wealth. Government subsidies to industry may have started with the dye industry: Louis Philippe of France decreed that soldiers in the French army were to wear trousers dyed with Turkey red. Well over a hundred years before, James II of England had banned the exportation of undyed cloth to protect English dyers.
The process of dyeing with natural dyes did not always produce consistent results and was often laborious and time consuming. But Turkey red, when it was obtained, was a beautiful bright red and very colorfast. The chemistry of the process was not understood, and today some of the operations involved seem somewhat bizarre and were probably unnecessary. Of the ten individual steps recorded in dyers' handbooks of the time, many are repeated more than once. At various stages the fabric or yarn is boiled in potash and in soap solution; mordanted with olive oil, alum, and a little chalk; treated with sheep dung, with tanning material, and with a tin salt; and rinsed overnight in river water, in addition to being dyed with madder.
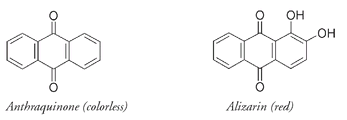
We now know the structure of the alizarin molecule that is responsible for the color of Turkey red and other shades from the madder plant. Alizarin is a derivative of anthraquinone, the parent compound of a number of naturally occurring coloring materials. More than fifty anthraquinone-based compounds have been found in insects, plants, fungi, and lichens. As with indigo, the parent anthraquinone is not colored. But the two OH groups on the right-hand ring in alizarin, combined with alternating double and single bonds in the rest of the molecule, provide enough conjugation for alizarin to absorb visible light.
OH groups are more important for producing color in these compounds than the number of rings. This is shown as well in compounds derived from naphthoquinone, a molecule with two rings compared to anthraquinone's three.
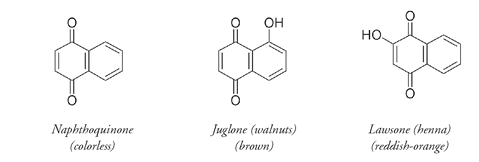
The naphthoquinone molecule is colorless; colored derivatives of naphthoquinone include
juglone,
found in walnuts, and
lawsone,
the coloring matter in Indian henna (used for centuries as a hair and skin dye). Colored naphthoquinones can have more than one OH group, as shown by
echinochrome,
a red pigment found in sand dollars and sea urchins.
juglone,
found in walnuts, and
lawsone,
the coloring matter in Indian henna (used for centuries as a hair and skin dye). Colored naphthoquinones can have more than one OH group, as shown by
echinochrome,
a red pigment found in sand dollars and sea urchins.
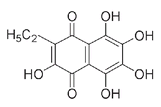
Echinochrome (red)
Another anthraquinone derivative, chemically similar to alizarin, is carminic acid, the principal dye molecule from cochineal, the other red dye from ancient times. Obtained from the crushed bodies of the female cochineal beetle,
Dactylopius coccus,
carminic acid contains numerous OH groups.
Dactylopius coccus,
carminic acid contains numerous OH groups.
Other books
The Poisoning in the Pub by Simon Brett
The Way of Wyrd by Brian Bates
South of Heaven by Ali Spooner
When Michael Met Mina by Randa Abdel-Fattah
Royal Outlaw: (Royal Outlaw, Book 1) by Kayla Hudson
Archie's Battleflat Adventures: The Harriman Mystery by King, Rebecca
Plagued by Barnett, Nicola
The Vespertine by Saundra Mitchell
The Pepper In The Gumbo: A Cane River Romance by Hathaway, Mary Jane
The Last Girls by Lee Smith