Penny le Couteur & Jay Burreson (27 page)
Read Penny le Couteur & Jay Burreson Online
Authors: Napoleon's Buttons: How 17 Molecules Changed History
Tags: #Philosophy & Social Aspects, #Science, #General, #World, #Chemistry, #Popular Works, #History

This polymer is today known as
polystyrene
and is used for plastic films, packing materials, and “Styrofoam” coffee cups. Styreneâprepared synthetically as early as 1866âand butadiene were the starting materials used by the German chemical company IG Farben in the manufacture of artificial rubber. The ratio of butadiene (CH
2
=CH-CH=CH
2
) to styrene is about three to one in SBR; though the exact ratio and structure are variable, it is thought that the double bonds are randomly cis or trans.
polystyrene
and is used for plastic films, packing materials, and “Styrofoam” coffee cups. Styreneâprepared synthetically as early as 1866âand butadiene were the starting materials used by the German chemical company IG Farben in the manufacture of artificial rubber. The ratio of butadiene (CH
2
=CH-CH=CH
2
) to styrene is about three to one in SBR; though the exact ratio and structure are variable, it is thought that the double bonds are randomly cis or trans.
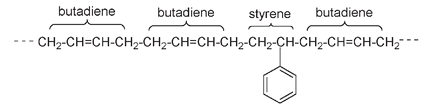
Partial structure of styrene butadiene rubber (SBR), also known as government rubber styrene (GR-S) or Buna-S. SBR can be vulcanized with sulfur.
In 1929 the Standard Oil Company of New Jersey formed a partnership with IG Farben based on shared processes relating to synthetic oil. Part of the agreement specified that Standard Oil would have access to certain of IG Farben's patents, including the SBR process. IG Farben was not obligated to share its technical details, however, and in 1938 the Nazi government informed the company that the United States was to be denied any information on Germany's advanced rubber-manufacturing technology.
IG Farben did eventually release the SBR patent to Standard Oil, certain that it contained insufficient technical information for the Americans to use in making their own rubber. But this judgment proved wrong. The chemical industry in the United States mobilized, and development of an SBR manufacturing process proceeded rapidly. In 1941 American synthetic rubber production was only eight thousand tons, but by 1945 it had expanded to over 800,000 tons, a significant proportion of the country's total rubber consumption. The production of such huge quantities of rubber in such a short period of time has been described as the second greatest feat of engineering (and chemistry) of the twentieth century, after the building of the atomic bomb. Over the following decades other synthetic rubbers (neoprene, butyl rubber, and Buna-N) were created. The meaning of the word
rubber
came to include polymers made from starting materials other than isoprene but with properties closely related to those of natural rubber.
rubber
came to include polymers made from starting materials other than isoprene but with properties closely related to those of natural rubber.
In 1953, Karl Ziegler in Germany and Giulio Natta in Italy further refined the production of synthetic rubber. Ziegler and Natta independently developed systems that produced either cis or trans double bonds depending on the particular catalyst used. It was now possible to make natural rubber synthetically. The so-called Ziegler-Natta catalysts, for which their discoverers received the 1963 Nobel Prize in chemistry, revolutionized the chemical industry by allowing the synthesis of polymers whose properties could be precisely controlled. In this way rubber polymers could be made that were more flexible, stronger, more durable, stiffer, less likely to be affected by solvents or ultraviolet light, and with greater resistance to cracking, heat, and cold.
Â
Â
Our world has been shaped by rubber. The collecting of raw material for rubber products had an enormous effect on society and the environment. The felling of the rubber trees in the Amazon basin, for example, was just one episode in the exploitation of the resources of tropical rain forests and destruction of a unique environment. The shameful treatment of the area's indigenous population has not changed; today prospectors and subsistence farmers continue to invade the traditional lands of the descendants of the native peoples who harvested latex. The brutal colonization of the Belgian Congo left a legacy of instability, violence, and strife that is still very much present in the region today. The mass migrations of workers to the rubber plantations of Asia over a century ago continue to affect the ethnic, cultural, and political face of the countries of Malaysia and Sri Lanka.
Our world is still shaped by rubber. Without rubber the enormous changes brought on by mechanization would not have been possible. Mechanization requires essential natural or man-made rubber components for machinesâbelts, gaskets, joints, valves, o-rings, washers, tires, seals, and countless others. Mechanized transportationâcars, trucks, ships, trains, planesâhas changed the way we move people and goods. Mechanization of industry has changed the jobs we do and the way we do them. Mechanization of agriculture has allowed the growth of cities and changed our society from rural to urban. Rubber has played an essential part in all these events.
Our exploration of future worlds may be shaped by rubber, as this materialâan essential part of space stations, space suits, rockets and shuttlesâis now enabling us to explore worlds beyond our own. But our failure to consider long-known properties of rubber has already limited our push to the stars. Despite NASA's sophisticated knowledge of polymer technology, rubber's lack of resistance to coldâa trait known to La Condamine, to Macintosh, to Goodyearâdoomed the space shuttle
Challenger
on a chilly morning in January 1986. The launch temperature was 36°F, 15° lower than the next-coldest previous launch. On the shuttle system's solid rocket motor aft field joint, the rubber o-ring in the shade on the side away from the sun was possibly as cold as 28°F. At that frigid temperature it would have lost its normal pliability and, by not returning to its proper shape, led to failure of a pressure seal. The resulting combustion gas leak caused an explosion that took the lives of the seven
Challenger
astronauts. This is a very recent example of what we might now call the Napoleon's buttons factor, the neglect of a known molecular property being responsible for a major tragic event:
“And all for the want of an O-ring.”
Challenger
on a chilly morning in January 1986. The launch temperature was 36°F, 15° lower than the next-coldest previous launch. On the shuttle system's solid rocket motor aft field joint, the rubber o-ring in the shade on the side away from the sun was possibly as cold as 28°F. At that frigid temperature it would have lost its normal pliability and, by not returning to its proper shape, led to failure of a pressure seal. The resulting combustion gas leak caused an explosion that took the lives of the seven
Challenger
astronauts. This is a very recent example of what we might now call the Napoleon's buttons factor, the neglect of a known molecular property being responsible for a major tragic event:
“And all for the want of an O-ring.”
9. DYES
D
YES COLOR OUR clothes, our furnishings, our accessories, and even our hair. Yet even as we ask for a different shade, a brighter hue, a softer tint, or a deeper tone, we rarely give a passing thought to the variety of compounds that allow us to indulge our passion for color. Dyes and dyestuffs are composed of natural or man-made molecules whose origins stretch back thousands of years. The discovery and exploitation of dyes has led to the creation and growth of the biggest chemical companies in the world today.
YES COLOR OUR clothes, our furnishings, our accessories, and even our hair. Yet even as we ask for a different shade, a brighter hue, a softer tint, or a deeper tone, we rarely give a passing thought to the variety of compounds that allow us to indulge our passion for color. Dyes and dyestuffs are composed of natural or man-made molecules whose origins stretch back thousands of years. The discovery and exploitation of dyes has led to the creation and growth of the biggest chemical companies in the world today.
The extraction and preparation of dyestuffs, mentioned in Chinese literature as long ago as 3000 B.C., may have been man's earliest attempts at the practice of chemistry. Early dyes were obtained mainly from plants: their roots, leaves, bark, or berries. Extraction procedures were well established and often quite complicated. Most substances did not adhere permanently to untreated fibers; fabrics first had to be treated with mordants, compounds that helped fix the color to the fiber. Although early dyes were highly sought after and very valuable, there were numerous problems with their use. They were frequently difficult to obtain, their range was limited, and the colors were not strong or faded quickly to dull, muddy shades in sunlight. Early dyes were rarely colorfast, bleeding out at every wash.
PRIMARY COLORSBlue, in particular, was a much-sought-after color. Compared with red and yellow, blue shades are not common in plants, but one plant,
Indigofera tinctoria,
a member of the legume family, was known to be a major source of the blue dye indigo. Named by the famed Swedish botanist Linnaeus,
Indigofera tinctoria
grows up to six feet tall in both tropical and subtropical climates. Indigo is also produced in more temperate regions from
Isatis tinctoria,
one of the oldest dye plants of Europe and Asia, known as “woad” in Britain and “pastel” in France. On his travels seven hundred years ago, Marco Polo was reputed to have seen indigo being used in the Indus valley; hence the name
indigo.
But indigo was also prevalent in many other parts of the world, including Southeast Asia and Africa, well before the time of Marco Polo.
Indigofera tinctoria,
a member of the legume family, was known to be a major source of the blue dye indigo. Named by the famed Swedish botanist Linnaeus,
Indigofera tinctoria
grows up to six feet tall in both tropical and subtropical climates. Indigo is also produced in more temperate regions from
Isatis tinctoria,
one of the oldest dye plants of Europe and Asia, known as “woad” in Britain and “pastel” in France. On his travels seven hundred years ago, Marco Polo was reputed to have seen indigo being used in the Indus valley; hence the name
indigo.
But indigo was also prevalent in many other parts of the world, including Southeast Asia and Africa, well before the time of Marco Polo.
The fresh leaves of indigo-producing plants do not appear to be blue. But after fermentation under alkaline conditions followed by oxidation, the blue color appears. This process was discovered by numerous cultures around the world, possibly when the plant leaves were accidentally soaked in urine or covered with ashes, then left to ferment. In these circumstances the conditions necessary for production of the intense blue color of indigo would be present.
The indigo precursor compound, found in all indigo-producing plants, is
indican,
a molecule that contains an attached glucose unit. Indican itself is colorless, but fermentation under alkaline conditions splits off the glucose unit to produce the
indoxol
molecule. Indoxol reacts with oxygen from the air to produce blue-colored indigo (or indigotin, as chemists call this molecule).
indican,
a molecule that contains an attached glucose unit. Indican itself is colorless, but fermentation under alkaline conditions splits off the glucose unit to produce the
indoxol
molecule. Indoxol reacts with oxygen from the air to produce blue-colored indigo (or indigotin, as chemists call this molecule).
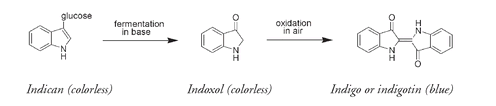
Indigo was a very valuable substance, but the most expensive of the ancient dyes was a very similar molecule known as Tyrian purple. In some cultures the wearing of purple was restricted by law to the king or emperor; hence the other name for this dyeâroyal purpleâand the phrase “born to the purple,” implying an aristocratic pedigree. Even today purple is still regarded as an imperial color, an emblem of royalty. Mentioned in writings dating to around 1600 B.C., Tyrian purple is the dibromo derivative of indigo; that is, an indigo molecule that contains two bromine atoms. Tyrian purple was obtained from an opaque mucus secreted by various species of a marine mollusk or snail, most commonly of the genus
Murex.
The compound secreted by the mollusk is, as in the indigo plant, attached to a glucose unit. It is only through oxidation in the air that the brilliant color of Tyrian purple develops.
Murex.
The compound secreted by the mollusk is, as in the indigo plant, attached to a glucose unit. It is only through oxidation in the air that the brilliant color of Tyrian purple develops.
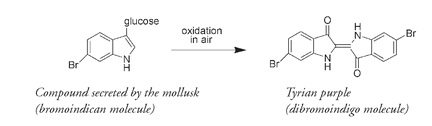
Bromine is rarely found in terrestrial plants or animals, but as there is a lot of bromine, as well as chlorine and iodine, in seawater, it is not that surprising to find bromine incorporated into compounds from marine sources. What is perhaps surprising is the similarity of these two molecules, given their very different sourcesâthat is, indigo from a plant and Tyrian purple from an animal.
Mythology credits the discovery of Tyrian purple to the Greek hero Hercules, who observed his dog's mouth becoming stained a deep purple color as the animal crunched on some shellfish. The manufacture of the dye is believed to have started in the Mediterranean port city of Tyre in the Phoenician Empire (now part of Lebanon). An estimated nine thousand shellfish were needed to produce one gram of Tyrian purple. Mounds of shells from
Murex brandaris
and
Purpura haemastoma
can still be found on the beaches of Tyre and Sidon, another Phoenician city involved in the ancient dye trade.
Murex brandaris
and
Purpura haemastoma
can still be found on the beaches of Tyre and Sidon, another Phoenician city involved in the ancient dye trade.
To obtain the dye, workers cracked open the shell of these mollusks and, using a sharp stick, extracted a small veinlike gland. Cloth was saturated with a treated solution from this gland, then exposed to the air for the color to develop. Initially the dye would turn cloth a pale yellow-green shade, then gradually blue, and then a deep purple. Tyrian purple colored the robes of Roman senators, Egyptian pharaohs, and European nobility and royalty. It was so sought after that by A.D. 400 the species of shellfish that produced it were in danger of becoming extinct.
Other books
The Ox-Bow Incident by Walter Van Tilburg Clark
El invierno en Lisboa by Antonio Muñoz Molina
Erotic Encounters by Gentry, Samantha
Stiletto Secrets by Bella J.
Bound by Lust by Shanna Germain
The Hidden Harbor Mystery by Franklin W. Dixon
As Dead as It Gets by Katie Alender
The Shattered Dark by Sandy Williams
Perfection #3 by Claire Adams
Halfstone: A Tale of the Narathlands by Daniel White