Penny le Couteur & Jay Burreson (23 page)
Read Penny le Couteur & Jay Burreson Online
Authors: Napoleon's Buttons: How 17 Molecules Changed History
Tags: #Philosophy & Social Aspects, #Science, #General, #World, #Chemistry, #Popular Works, #History

With his financial problem solved, Baekeland turned his attention to creating a synthetic version of shellac, a material that had been used for many years as a lacquer and wood preservative and is still used today. Shellac is obtained from an excretion of the female lac or
Laccifer lacca
beetle, a native of Southeast Asia. The beetles attach themselves to trees, suck the sap, and eventually become encased in a covering of their own secretion. After reproducing, the beetles die, and their cases or shellsâhence the
shell
part of the word
shellac
âare gathered and melted. The liquid thus obtained is filtered to remove the bodies of the dead beetles. It takes fifteen thousand lac beetles six months to produce a single pound of shellac. As long as the shellac was used only as a thin coating, the price was affordable, but with increased use of shellac by the electrical industry, which was rapidly expanding at the beginning of the twentieth century, the demand for shellac soared. The cost of making an electrical insulator, even just using shellac-impregnated paper, was high, and Baekeland realized that artificial shellac would become a necessity for insulators in this increasing market.
Laccifer lacca
beetle, a native of Southeast Asia. The beetles attach themselves to trees, suck the sap, and eventually become encased in a covering of their own secretion. After reproducing, the beetles die, and their cases or shellsâhence the
shell
part of the word
shellac
âare gathered and melted. The liquid thus obtained is filtered to remove the bodies of the dead beetles. It takes fifteen thousand lac beetles six months to produce a single pound of shellac. As long as the shellac was used only as a thin coating, the price was affordable, but with increased use of shellac by the electrical industry, which was rapidly expanding at the beginning of the twentieth century, the demand for shellac soared. The cost of making an electrical insulator, even just using shellac-impregnated paper, was high, and Baekeland realized that artificial shellac would become a necessity for insulators in this increasing market.
Baekeland's first approach to the problem of making a shellac involved the reaction of phenolâthe same molecule with which Lister had successfully transformed surgeryâand formaldehyde, a compound derived from methanol (or wood alcohol) and in those days used extensively as an embalming agent by undertakers and for preserving animal specimens.

Previous attempts to combine these compounds had produced discouraging results. Rapid, uncontrolled reactions led to insoluble and infusible materials that were too brittle and inflexible to be useful. But Baekeland recognized that such properties could be just what was needed in a synthetic shellac for electrical insulators, if only he could control the reaction so the material could be processed into a usable form.
By 1907, using a reaction in which he was able to control both heat and pressure, Baekeland had produced a liquid that rapidly hardened into a transparent, amber-colored solid conforming exactly to the shape of the mold or vessel into which it was poured. He named the material
Bakelite
and called the modified pressure cooker-like device used to produce it the
Bakelizer.
We can perhaps excuse the self-promotion inherent in these names when we consider that Baekeland had spent five years working with this one reaction in order to synthesize this substance.
Bakelite
and called the modified pressure cooker-like device used to produce it the
Bakelizer.
We can perhaps excuse the self-promotion inherent in these names when we consider that Baekeland had spent five years working with this one reaction in order to synthesize this substance.
While shellac would become distorted with heat, Bakelite retained its shape at high temperatures. Once set, it could not be melted and remolded. Bakelite was a
thermoset
material; that is, it was frozen in its shape forever, as opposed to a
thermoplastic
material like celluloid. This phenolic resin's unique thermoset property was due to its chemical structure: formaldehyde in Bakelite can react at three different places on the benzene ring of phenol, causing cross-links among the polymer chains. Rigidity in Bakelite is attributed to these very short cross-links attached to already rigid and planar benzene rings.
thermoset
material; that is, it was frozen in its shape forever, as opposed to a
thermoplastic
material like celluloid. This phenolic resin's unique thermoset property was due to its chemical structure: formaldehyde in Bakelite can react at three different places on the benzene ring of phenol, causing cross-links among the polymer chains. Rigidity in Bakelite is attributed to these very short cross-links attached to already rigid and planar benzene rings.
Schematic formula for Bakelite showing -CH2- cross-links between phenol molecules. These are only some possible ways of linking; in the actual material the linkages are random.
When used for electrical insulators, Bakelite's performance was superior to that of any other material. It was more heat resistant than shellac or any shellac-impregnated paper versions; it was less brittle than ceramic or glass insulators; and it had better electrical resistance than porcelain or mica. Bakelite did not react with sun, water, salt air, or ozone and was impervious to acid and solvents. It didn't easily crack, chip, discolor, fade, burn, or melt.
Subsequently, though not the original intent of its inventor, Bakelite was found to be the ideal material for billiard balls. Bakelite's elasticity was very similar to that of ivory, and Bakelite billiard balls, when they collided, made the same agreeable clicking sound as did ivory billiard balls, an important factor that was lacking in celluloid versions. By 1912 almost all nonivory billiard balls were made of Bakelite. Numerous other applications followed, and within the space of a few years Bakelite was everywhere. Telephones, bowls, washing machine agitators, pipe stems, furniture, car parts, fountain pens, plates, glasses, radios, cameras, kitchen equipment, handles for knives, brushes, drawers, bathroom fittings, and even artworks and decorative items were all being made of Bakelite. Bakelite became known as the “material for a thousand uses”âthough nowadays other phenolic resins have superseded the original brown material. Later resins were colorless and could be easily tinted.
A PHENOL FOR FLAVORThe creation of Bakelite is not the only example where a phenol molecule was the basis of the development of an artificial substance to take the place of a natural substance for which demand had exceeded supply. The market for vanillin has long surpassed the supply available from the vanilla orchid. So synthetic vanillin is manufactured and comes from a surprising source: the waste pulp liquor from the sulfite treatment of wood pulp in the making of paper. Waste liquor consists mainly of lignin, a substance found in and between the cell walls of land plants. Lignin contributes to the rigidity of plants and makes up about 25 percent of the dry weight of wood. It is not one compound but a variable cross-bonded polymer of different phenolic units.
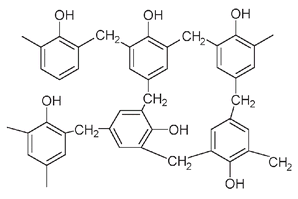
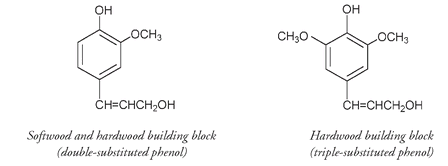
There is a difference in the lignin composition between softwoods and hardwoods as shown by the structures of the building blocks of their respective lignins. In lignin, as in Bakelite, the rigidity of wood depends on the degree of cross-linking between phenolic molecules. The triple-substituted phenols, found only in hardwoods, allow for more cross-linkages and thus explain why hardwoods are “harder” than softwoods.
A representative structure for lignin showing some of the cross-links between these building-block units is illustrated below. It has definite similarities to Baekeland's Bakelite.
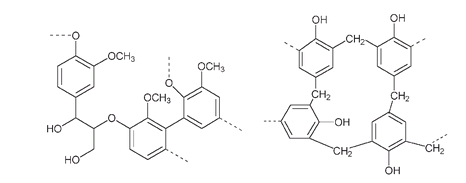
Part of the structure of lignin (left). The dotted lines indicate connection to the rest of the molecule. The structure of Bakelite (right) also has cross-links between phenol units.
The circled part in the drawing of lignin (below) highlights part of the structure that is very similar to the vanillin molecule. When a lignin molecule is broken up under controlled conditions, vanillin can be produced.
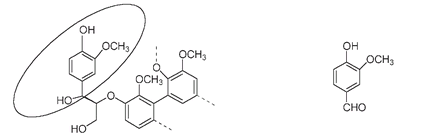
Lignin (left), with the circled part of its structure very similar to the vanillin molecule (right)
Synthetic vanillin is not just a chemical imitation of the real thing; rather, it is pure vanillin molecules made from a natural source and is chemically absolutely identical to vanillin from the vanilla bean. Vanilla flavoring obtained from using the whole vanilla bean does, however, contain trace amounts of other compounds that, together with the vanillin molecule, give the overall flavor of true vanilla. Artificial vanilla flavoring contains synthetic vanillin molecules in a solution containing caramel as a coloring agent.
Odd as it may sound, there is a chemical connection between vanilla and the phenol molecule, found as carbolic acid. Under great pressures and moderate temperatures over a long time, coal forms from decomposing plant materials, including, of course, lignin from woody tissues, as well as cellulose, another major component of vegetation. In the process of heating coal to obtain the important coal gas fuel for homes and industries, a black viscous liquid with an acrid smell is obtained. This is coal tar, Lister's source of carbolic acid. His antiseptic phenol was ultimately derived from lignin.
It was phenol that first permitted antiseptic surgery, allowing operations to be performed without risk of life-threatening infection. Phenol changed the prospects of survival for thousands injured in accidents or wars. Without phenol and later antiseptics the amazing surgical feats of todayâhip replacements, open-heart surgery, organ transplants, neurosurgery, and microsurgical repairsâwould never have been possible.
By investing in Baekeland's invention for photographic paper, George Eastman was able to offer a better film that, together with the introduction in 1900 of a very inexpensive cameraâthe Kodak Brownie, which sold for one dollarâchanged photography from a pursuit of the wealthy to a hobby available to everyone. Eastman's investment financed the developmentâwith phenol as a starting materialâof the first truly synthetic material of the Age of Plastics, Bakelite, used to make the insulators necessary for the widespread use of electrical energy, a major factor in the modern industrial world.
The phenols we have discussed have changed our lives in many large ways (antiseptic surgery, the development of plastics, explosive phenols) and in many small ways (potential health factors, spicy foods, natural dyes, affordable vanilla). With such a wide variety of structures it is likely that phenols will continue to shape history.
8. ISOPRENE
C
AN YOU IMAGINE what the world would be like without tires for our cars and trucks and planes? Without gaskets and fan belts for our engines, elastic in our clothes, waterproof soles for our shoes? Where would we be without such mundane but useful items as rubber bands?
AN YOU IMAGINE what the world would be like without tires for our cars and trucks and planes? Without gaskets and fan belts for our engines, elastic in our clothes, waterproof soles for our shoes? Where would we be without such mundane but useful items as rubber bands?
Other books
Patiently Alice by Phyllis Reynolds Naylor
Lord Malquist & Mr. Moon: A Novel by Tom Stoppard
Honeymooning by Rachael Herron
Somewhere Out There by Amy Hatvany
The Charred Lands: Apocalypse of Fire by Josh A. Murphy
Killer Kitchens (Murders by Design) by Harrington, Jean
Devil's Sin by Kathryn Thomas
The Woken Gods by Gwenda Bond
MiNRS by Kevin Sylvester