Penny le Couteur & Jay Burreson (21 page)
Read Penny le Couteur & Jay Burreson Online
Authors: Napoleon's Buttons: How 17 Molecules Changed History
Tags: #Philosophy & Social Aspects, #Science, #General, #World, #Chemistry, #Popular Works, #History


Women rushed to buyâand wearânylons after World War II when the polymer became available for hosiery again.
(Photo courtesy of Du Pont)
(Photo courtesy of Du Pont)
7. PHENOL
T
HE VERY FIRST totally man-made polymer was produced about twenty-five years before Du Pont's nylon. It was a somewhat random cross-linked material made from a compound whose chemical structure was similar to some of the spice molecules to which we attributed the Age of Discovery. This compound, phenol, started another age, the Age of Plastics. Linked to such diverse topics as surgical practices, endangered elephants, photography, and orchids, phenols have played a pivotal role in a number of advances that have changed the world.
STERILE SURGERYHE VERY FIRST totally man-made polymer was produced about twenty-five years before Du Pont's nylon. It was a somewhat random cross-linked material made from a compound whose chemical structure was similar to some of the spice molecules to which we attributed the Age of Discovery. This compound, phenol, started another age, the Age of Plastics. Linked to such diverse topics as surgical practices, endangered elephants, photography, and orchids, phenols have played a pivotal role in a number of advances that have changed the world.
In 1860 you would not have wanted to be a patient in a hospitalâespecially not to undergo an operation. Hospitals were dark, grimy, and airless. Patients were commonly given beds where the bedclothes were not changed after the previous occupant leftâor more probably died. Surgical wards exuded an appalling stench from gangrene and sepsis. Equally appalling was the death rate from such bacterial infections; at least 40 percent of amputees died from so-called hospital disease. In army hospitals that number approached 70 percent.
Despite the fact that anesthetics had been introduced at the end of 1864, most patients agreed to surgery only as a last resort. Surgical wounds always became infected; accordingly, a surgeon would ensure that the stitches closing an operation site were left long, hanging down toward the ground, so that pus could drain away from the wound. When this happened, it was considered a positive sign, as chances were good that the infection would stay localized and not invade the rest of the body.
Of course, we now know why “hospital disease” was so prevalent and so lethal. It was actually a group of diseases caused by a variety of bacteria that easily passed from patient to patient or even from doctor to a series of patients under unsanitary conditions. When hospital disease became too rife, a physician usually closed down his surgical ward, sent the remaining patients elsewhere, and had the premises fumigated with sulfur candles, the walls whitewashed, and the floors scrubbed. For a while after these precautions infections would be under controlâuntil another outbreak required further attention.
Some surgeons insisted on maintaining constant strict cleanliness, a regime involving lots of cooled boiled water. Others supported the miasma theory, the belief that a poisonous gas generated by drains and sewers was carried in the air and that once a patient was infected, this miasma was transferred through the air to other patients. This miasma theory probably seemed very reasonable at the time. The stench from drains and sewers would have been as bad as the smell of putrefying gangrenous flesh in surgery wards, which would further explain how patients treated at home rather than in a hospital often escaped infection altogether. Various treatments were prescribed to counteract miasma gases, including thymol, salicylic acid, carbon dioxide gas, bitters, raw carrot poultices, zinc sulfate, and boracic acid. The occasional success of any of these remedies was fortuitous and could not be replicated at will.
This was the world in which the physician Joseph Lister was practicing surgery. Born in 1827 to a Quaker family from Yorkshire, Lister completed his medical degree at University College in London, and by 1861 was a surgeon at the Royal Infirmary in Glasgow and a professor of surgery at the University of Glasgow. Though a new modern surgical block had been opened at the Royal Infirmary during Lister's tenure, hospital disease was just as much a problem there as it was elsewhere.
Lister believed its cause might not be a poisonous gas but something else in the air, something that could not be seen with the human eye, something microscopic. On reading a paper that described “The Germ Theory of Diseases,” he immediately recognized its applicability to his own ideas. The paper had been written by Louis Pasteur, a professor of chemistry in Lille, northeastern France, and mentor to Chardonnet of Chardonnet silk fame. Pasteur's experiments on the souring of wine and milk had been presented to a gathering of scientists at the Sorbonne in Paris in 1864. Germsâmicroorganisms that could not be detected by the human eyeâwere considered by Pasteur to be everywhere. His experiments showed that such microorganisms could be eliminated by boiling, leading of course to our present-day pasteurization of milk and other foodstuffs.
As boiling patients and surgeons was not practical, Lister had to find some other way to safely eliminate germs on all surfaces. He settled on carbolic acid, a product made from coal tar that had been used successfully to treat stinking city drains and that had already been tried as a dressing on surgical wounds, without very positive results. Lister persevered and met with success in the case of an eleven-year-old boy who came to the Royal Infirmary with a compound fracture of the leg. At the time compound fractures were a dreaded injury. A simple fracture could be set without invasive surgery, but a compound fracture, where the sharp ends of broken bones had pierced the skin, was almost certain to become infected, despite a surgeon's skill at setting the bone. Amputation was a common outcome, and death from an uncontrollable persistent infection was likely.
Lister carefully cleaned the area in and around the boy's broken bone with lint soaked in carbolic acid. Then he prepared a surgical dressing consisting of layers of linen soaked in carbolic solution and covered with a thin metal sheet bent over the leg to reduce possible evaporation of the carbolic acid. This dressing was carefully taped in place. A scab soon formed, the wound healed rapidly, and infection never occurred.
Other patients had survived their infections from hospital disease, but this was a case in which infection had been prevented, not just outlasted. Lister treated subsequent compound fracture cases the same way, producing the same positive outcome, convincing him of the effectiveness of carbolic solutions. By August 1867 he was using carbolic acid as an antiseptic agent during all his surgical procedures, not just as a postoperative dressing. He improved his antiseptic techniques over the next decade, gradually convincing other surgeons, many of whom still refused to believe in the germ theory as “if you can't see them, they are not there.”
Coal tar, from which Lister obtained his carbolic acid solution, was readily available as a waste product from the gaslight illumination of city streets and houses during the nineteenth century. The National Light and Heat Company had installed the first gas street lighting in Westminster, London, in 1814, and widespread use of gas lighting followed in other cities. Coal gas was produced by heating coal at high temperatures; it was a flammable mixtureâabout 50 percent hydrogen, 35 percent methane, and smaller amounts of carbon monoxide, ethylene, acetylene, and other organic compounds. It was piped to homes, factories, and streetlamps from local gasworks. As demand for coal gas grew, so did the problem of what to do with coal tar, the seemingly unimportant residue of the coal-gasification process.
Coal tar was a viscous, black, acrid-smelling liquid that would eventually prove an amazingly prolific source for a number of important aromatic molecules. Not until huge reservoirs of natural gas, consisting mainly of methane, were discovered in the early part of the twentieth century did the coal-gasification process and its accompanying production of coal tar decline. Crude carbolic acid, as first used by Lister, was a mixture distilled from coal tar at temperatures between 170°C and 230°C. It was a dark and very strong-smelling oily material that burned the skin. Lister was eventually able to obtain the main constitutent of carbolic acid,
phenol,
in its pure form as white crystals.
phenol,
in its pure form as white crystals.
Phenol is a simple aromatic molecule consisting of a benzene ring, to which is attached an oxygen-hydrogen or OH group.
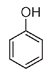
Phenol
It is somewhat water soluble and is very soluble in oil. Lister made use of these characteristics by developing what became known as the “carbolic putty poultice,” a mixture of phenol with linseed oil and whitening (powdered chalk). The resultant paste (spread on a sheet of tinfoil) was placed poultice side down on the wound and acted like a scab, providing a barrier to bacteria. A less concentrated solution of phenol in water, usually about one part of phenol to between twenty and forty parts of water, was used to wash the skin around a wound, the surgical instruments, and the surgeon's hands, and it was also sprayed onto an incision during an operation.
Despite the effectiveness of his carbolic acid treatment, as shown by the recovery rates of his patients, Lister was not satisfied that he had achieved totally antiseptic conditions during surgical operations. He thought that every dust particle in the air bore germs, and in an effort to prevent these airborne germs from contaminating operations, he developed a machine that continually sprayed a fine mist of carbolic acid solution into the air, effectively drenching the whole area. Airborne germs are actually far less a problem than Lister had assumed. The real issue was microorganisms that came from the clothes, hair, skin, mouths, and noses of the surgeons, the other doctors, and the medical students who routinely assisted with or watched operations without taking any antiseptic precautions. Modern operating-room protocol of sterile masks, scrub suits, hair coverings, drapes, and latex gloves now solves this problem.
Lister's carbolic spray machine did help prevent contamination from microorganisms, but it had negative effects on the surgeons and others in the operating room. Phenol is toxic, and even in dilute solutions, it causes bleaching, cracking, and numbing of the skin. Inhaling phenolic spray can lead to illness; some surgeons refused to continue working when a phenol spray was in use. Despite these drawbacks Lister's techniques of antiseptic surgery were so effective and the positive results so obvious that by 1878 they were in use throughout the world. Phenol is rarely used today as an antiseptic; its harsh effect on the skin and its toxicity make it less useful than newer antiseptics that have been developed.
MANY-FACETED PHENOLSThe name
phenol
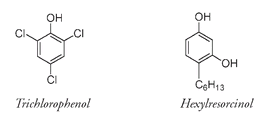
does not apply only to Lister's antiseptic molecule; it is applied to a very large group of related compounds that all have an OH group attached directly to a benzene ring. This may seem a bit confusing, as there are thousands or even hundreds of thousands of phenols, but only one “phenol.” There are man-made phenols, like trichlorophenol and hexylresorcinols, with antibacterial properties that are used today as antiseptics.
phenol
does not apply only to Lister's antiseptic molecule; it is applied to a very large group of related compounds that all have an OH group attached directly to a benzene ring. This may seem a bit confusing, as there are thousands or even hundreds of thousands of phenols, but only one “phenol.” There are man-made phenols, like trichlorophenol and hexylresorcinols, with antibacterial properties that are used today as antiseptics.
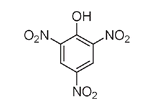
Picric acid, originally used as a dyeâespecially for silkâand later in armaments by the British in the Boer War and in the initial stages of World War I, is a triple-nitrated phenol and is highly explosive.
Other books
Taken by the Warrior King by Vanessa E. Silver
A Thousand Splendid Suns by Khaled Hosseini
A Measure of Love by Sophie Jackson
Walk by Faith by Rosanne Bittner
Warped Passages by Lisa Randall
Vacation Under the Volcano by Mary Pope Osborne
The Champion by Scott Sigler
Scorpion Shards by Neal Shusterman
Maybe Tonight by Kim Golden
These High, Green Hills by Jan Karon