Penny le Couteur & Jay Burreson (18 page)
Read Penny le Couteur & Jay Burreson Online
Authors: Napoleon's Buttons: How 17 Molecules Changed History
Tags: #Philosophy & Social Aspects, #Science, #General, #World, #Chemistry, #Popular Works, #History

In 218 B.C., the Carthaginian general Hannibal made his way through the Alps with his vast army and his forty elephants for an assult on the heart of the Roman Empire. He used the standard but extremely slow road-building method of the day: rock obstacles were heated by bonfires, then doused with cold water to crack them apart. Had Hannibal possessed explosives, a rapid passage through the Alps might have allowed him an eventual victory at Rome, and the fate of the whole western Mediterranean would have been very different.
From Vasco da Gama's defeat of the rulers of Calicut, through the conquest of the Aztec empire by Hernán Cortés and a handful of Spanish conquistadors, to the British army's Light Cavalry Brigade charge of Russian field batteries in the 1854 Battle of Balaklava, explosive-propelled weapons have had the advantage over bows and arrows, spears, and swords. Imperialism and colonialismâsystems that have molded our worldâdepended on the power of armaments. In war and in peace, from destroying to constructing, for worse or for better, explosive molecules have changed civilization.
6. SILK AND NYLON
E
XPLOSIVE MOLECULES may seem very remote from the images of luxury, softness, suppleness, and sheen conjured up by the word
silk.
But explosives and silk have a chemical connection, one that led to the development of new materials, new textiles, and by the twentieth century, a whole new industry.
XPLOSIVE MOLECULES may seem very remote from the images of luxury, softness, suppleness, and sheen conjured up by the word
silk.
But explosives and silk have a chemical connection, one that led to the development of new materials, new textiles, and by the twentieth century, a whole new industry.
Silk has always been prized as a fabric by the wealthy. Even with the wide choice of both natural and man-made fibers available today, it is still considered irreplaceable. The properties of silk that have long made it so desirableâits caressing feel, its warmth in cool weather and coolness in hot weather, its wonderful luster, and the fact that it takes dyes so beautifullyâare due to its chemical structure. Ultimately it was the chemical structure of this remarkable substance that opened trade routes between the East and the rest of the known world.
THE SPREAD OF SILKThe history of silk goes back more than four and a half millennia. Legend has it that around 2640 B.C., Princess Hsi-ling-shih, the chief concubine of the Chinese emperor Huang-ti, found that a delicate thread of silk could be unwound from an insect cocoon that had fallen into her tea. Whether this story is myth or not, it is true that the production of silk started in China with the cultivation of the silkworm,
Bombyx mori,
a small gray worm that feeds only on the leaves of the mulberry tree,
Morus alba.
Bombyx mori,
a small gray worm that feeds only on the leaves of the mulberry tree,
Morus alba.
Common in China, the silkworm moth lays around five hundred eggs over a five-day period and then dies. One gram of these tiny eggs produces more than a thousand silkworms, all of which together devour about thirty-six kilograms of ripe mulberry leaves to produce around two hundred grams of raw silk. The eggs must initially be kept at 65°F, then gradually rise to the hatching temperature of 77°F. The worms are kept in clean, well-ventilated trays, where they eat voraciously and shed their skins several times. After a month they are moved to spinning trays or frames to start spinning their cocoons, a process that takes several days. A single continuous strand of silk thread is extruded from the worm's jaw, along with a sticky secretion that holds the threads together. The worm moves its head constantly in a figure-eight pattern, spinning a dense cocoon and gradually changing itself into a chrysalis.
To obtain the silk, the cocoons are heated to kill the chrysalis inside and then plunged into boiling water to dissolve the sticky secretion holding the threads together. Pure silk thread is then unwound from the cocoon and wound onto reels. The length of a silk thread from one cocoon can be anywhere from four hundred to more than three thousand yards.
The cultivation of silkworms and the use of the resulting silk fabric spread rapidly throughout China. Initially, silk was reserved for members of the imperial family and for nobility. Later, though its price remained high, even the common people were allowed to wear garments made of silk. Beautifully woven, lavishly embroidered, and wonderfully dyed, silk fabric was greatly prized. It was a highly valued commodity of trade and barter and even a form of currencyârewards and taxes were sometimes paid in silk.
For centuries, long after the opening of the trade routes through Central Asia collectively known as the Silk Road, the Chinese kept the details of silk production a secret. The path of the Silk Road varied over the centuries, depending mostly on the politics and safety of the regions along the way. At its longest it extended some six thousand miles from Peking (Beijing), in eastern China, to Byzantium (later Constantinople, now Istanbul) in present-day Turkey, and to Antioch and Tyre, on the Mediterranean, with major arteries diverting into northern India. Some parts of the Silk Road date back over four and a half millennia.
Trade in silk spread slowly, but by the first century B.C. regular shipments of silk were arriving in the West. In Japan sericulture began around A.D. 200 and developed independently from the rest of the world. The Persians quickly became middlemen in the silk trade. The Chinese, to maintain their monopoly on production, made attempts to smuggle silkworms, silkworm eggs, or white mulberry seeds from China punishable by death. But as the legend goes, in 552 two monks of the Nestorian church managed to return from China to Constantinople with hollowed-out canes that concealed silkworm eggs and mulberry seeds. This opened the door to silk production in the West. If the story is true, it is possibly the first recorded example of industrial espionage.
Sericulture spread throughout the Mediterranean and by the fourteenth century was a flourishing industry in Italy, especially in the north, where cities like Venice, Lucca, and Florence became renowned for beautiful heavy silk brocades and silk velvets. Silk exports from these areas to northern Europe are considered to be one of the financial bases of the Renaissance movement, which started in Italy about this time. Silk weavers fleeing political instability in Italy helped France become a force in the silk industry. In 1466, Louis XI granted tax exemptions for silk weavers in the city of Lyons, decreed the planting of mulberry trees, and ordered the manufacture of silk for the royal court. For the next five centuries European sericulture would be centered near Lyons and its surrounding area. Macclesfield and Spittalfield, in England, became major centers for finely woven silks when Flemish and French weavers, escaping religious persecution on the Continent, arrived in the late sixteenth century.
Various attempts to cultivate silk in North America were not commercially successful. But the spinning and weaving of silk, processes that could be readily mechanized, were developed. In the early part of the twentieth century the United States was one of the largest manufacturers of silk goods in the world.
THE CHEMISTRY OF SHEEN AND SPARKLESilk, like other animal fibers such as wool and hair, is a protein. Proteins are made from twenty-two different α-amino acids. The chemical structure for an α-amino acid has an amino group (NH
2
) and an organic acid group (COOH) arranged as shown, with the NH
2
group on the carbon atom of the alpha carbonâthat is, the carbon adjacent to the COOH group.
2
) and an organic acid group (COOH) arranged as shown, with the NH
2
group on the carbon atom of the alpha carbonâthat is, the carbon adjacent to the COOH group.
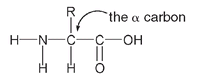
Generalized structure for an
α
-amino acid
α
-amino acid
This is often drawn more simply in its condensed version as
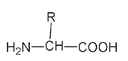

The condensed structure of the generalized amino acid structure
In these structures R represents a different group, or combination of atoms, for each amino acid. There are twenty-two different structures for R, and this is what makes the twenty-two amino acids. The R group is sometimes called the side group or the side chain. The structure of this side group is responsible for the special properties of silkâand indeed for the properties of any protein.
The smallest side group, and the only side group consisting of just one atom, is the hydrogen atom. Where this R group is H, the name of the amino acid is
glycine,
and the structure is as shown as follows.
glycine,
and the structure is as shown as follows.
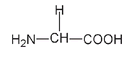
The amino acid glycine
Other simple side groups are CH
3
and CH
2
OH, giving the amino acids
alanine
and
serine
respectively.
3
and CH
2
OH, giving the amino acids
alanine
and
serine
respectively.

These three amino acids have the smallest side groups of all the amino acids, and they are also the most common amino acids in silk, constituting together about 85 percent of silk's overall structure. That the side groups in the silk amino acids are physically very small is an important factor in the smoothness of silk. In comparison, other amino acids have much larger, more complicated side groups.
Like cellulose, silk is a polymerâa macromolecule made up of repeating units. But unlike the cellulose polymer of cotton, in which the repeating units are exactly the same, the repeating units of protein polymers, amino acids, vary somewhat. The parts of the amino acid that form a polymer chain are all the same. It is the side group on each amino acid that differs.
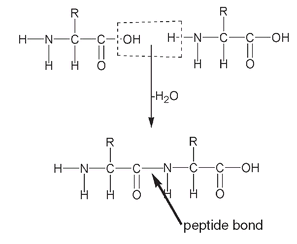
Two amino acids combine by eliminating water between themselves, an H atom from the NH
2
or amino end and an OH from the COOH or acid end. The resulting link between the two amino acids is known as an
amide group.
The actual chemical bond between the carbon of one amino acid and the nitrogen of the other amino acid is known as a
peptide bond.
2
or amino end and an OH from the COOH or acid end. The resulting link between the two amino acids is known as an
amide group.
The actual chemical bond between the carbon of one amino acid and the nitrogen of the other amino acid is known as a
peptide bond.
Other books
Shock Treatment by James Hadley Chase
The Firecracker Gets Her Man by Joannie Kay
Begin Again by Christy Newton
The Angelus Guns by Max Gladstone
Marry Me by Cheryl Holt
Odd Interlude #3 (An Odd Thomas Story) by Koontz, Dean
A Child's Book of True Crime by Chloe Hooper
Donut Days by Lara Zielin
Darius & Twig by Walter Dean Myers
Just Desserts by Valentine, Marquita